* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Toxicology of the Nervous System
Holonomic brain theory wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
End-plate potential wikipedia , lookup
Electrophysiology wikipedia , lookup
Aging brain wikipedia , lookup
Node of Ranvier wikipedia , lookup
Neurotransmitter wikipedia , lookup
Nervous system network models wikipedia , lookup
Optogenetics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Metastability in the brain wikipedia , lookup
Subventricular zone wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroregeneration wikipedia , lookup
Long-term depression wikipedia , lookup
Signal transduction wikipedia , lookup
Circumventricular organs wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Haemodynamic response wikipedia , lookup
Chemical synapse wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neuroanatomy wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
NMDA receptor wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
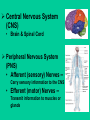
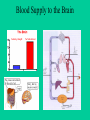
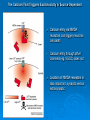
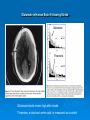
Neurotoxicity: Toxicology of the Nervous System John J Woodward, PhD Department of Neurosciences IOP471N [email protected] www.people.musc.edu/~woodward Historical Events 1930’s – Ginger-Jake Syndrome • During prohibition, an alcohol beverage was contaminated with TOCP (triortho cresyl phosphate) causing paralysis in 5,000 with 20,000 to 100,000 affected. 1950’s – Mercury poisoning • Methylmercury in fish in Japan cause death and severe nervous system damage in infants and adults (Minimata disease). Central Nervous System (CNS) • Brain & Spinal Cord Peripheral Nervous System (PNS) • Afferent (sensory) Nerves – Carry sensory information to the CNS • Efferent (motor) Nerves – Transmit information to muscles or glands Cells of the Nervous System Neurons • Signal integration/generation; direct control of skeletal muscle (motor axons) Supporting Cells (Glia cells) • • • • Astrocytes (CNS – blood brain barrier) Oligodendrocytes (CNS – myelination) Schwann cells (PNS – myelination) Microglia (activated astrocytes) Underlying Cellular Biology Cellular Events in Neurodevelopment Events: Division Migration Differentiation Neurogenesis Formation of synapses Myelination Apoptosis Active throughout childhood & adolescence Development of GABA and Glutamate Synapses in Primate Hippocampus •GABA synapses develop on contact •Glutamate synapses develop but require a developed spine to become active •GDPs dominate early developmental neuronal activity and disappear prior to birth (primates) or during early neonatal life (rodents) 6 Why is the Brain Particularly Vulnerable to Injury? Neurons are post-mitotic cells High dependence on oxygen • Little anaerobic capacity • Brief hypoxia/anoxia-neuron cell death Dependence on glucose • Sole energy source (no glycolysis) • Brief disruption of blood flow-cell death High metabolic rate Many substances go directly to the brain via inhalation Blood Supply to the Brain Blood-brain Barrier Anatomical Characteristics • • • Capillary endothelial cells are tightly joined – no pores between cells Capillaries in CNS surrounded by astrocytes Active ATP-dependent transporter – moves chemicals into the blood Not an absolute barrier • • • • Caffeine (small), nicotine Methylmercury cysteine complex Lipids (barbiturate drugs and alcohol) Susceptible to various damages BBB can be broken down by: • Hypertension: high blood pressure opens the BBB • Hyperosmolarity: high concentration of solutes can open the BBB. • Infection: exposure to infectious agents can open the BBB. • Trauma, Ischemia, Inflammation, Pressure: injury to the brain can open the BBB. • Development: the BBB is not fully formed at birth. What causes neurotoxicity? Wide range of causes Chemical Physical Toxicants and Exposure • Inhalation (e.g. solvents, nicotine, nerve gases) • Ingestions (e.g. lead, alcohol, drugs such as MPTP) • Skin (e.g. pesticides, nicotine) • Physical (e.g. load noise, trauma) NEURONS CELL MEMBRANE AND MEMBRANE PROTEINS Ion Channels •Important for establishing resting membrane potetial •Synaptic transmission/nerve conduction •Voltage-sensitive Sodium channel •Ligand-gated Types of Neurotoxic Injury Normal Neuron Myelin Axon Synapse Axonopathy Transmission Neuronopathy Myelinopathy Types Of Neurotoxicity Neuronopathy • • Cell Death. Irreversible – cells not replaced. MPTP, Trimethyltin Axonopathy • • Degeneration of axon. May be reversible. Hexane, Acrylamide, physical trauma Myelinopathy • • Damage to myelin (e.g. Schwann cells) Lead, Hexachlorophene Transmission Toxicity • Disruption of neurotransmission, toxins, heavy metals, organophosphate pesticides, DDT, drugs (eg., cocaine, amphetamine, alcohol) Ion Channels are Targets for a Variety of Toxins, Chemicals and Therapeutic Compounds Natural Toxins Snake, insect,plant toxins (cobra venom, scorpion, curare) Environmental Chemicals Heavy metals, industrial solvents (lead, benzene, aromatic hydrocarbons) Therapeutic Drugs Anesthetics, Benzodiazepines (lidocaine, halothane, valium) Drugs of abuse (Ketamine, alcohol, inhalants) Neurotoxicology Heavy Metals Lead – environmental exposure (paint, fuels) Mercury – exposure via diet (bioaccumulation in fish) Historical Sources of Lead Exposure Ancient/Premodern History • Lead oxide as a sweetening agent • Lead pipes (“plumbing”) • Ceramics • Smelting and foundries Modern History • Gasoline (leaded) • Ceramics • Crystal glass • Soldering – pipes – “tin” cans – car radiators • House paint Lead Neurotoxicity Nervous Systems Effects Developmental Neurotoxicity Reduced IQ Impaired learning and memory Life-long effects Related to effects on calcium permeable channels (NMDA, Ca++ channels) Mechanisms of Damage to the Nervous System by Lead Central • Cerebral edema • Apoptosis of neuronal cells • Necrosis of brain tissue • Glial proliferation around blood vessels Peripheral • Demyelination • Reversible changes in nerve conduction velocity (NCV) • Irreversible axonal degeneration Environmental Sources of Mercury • Natural Degassing of the earth • Combustion of fossil fuel • Industrial Discharges and Wastes • Incineration & Crematories • Dental amalgams • CF bulbs Toxicity of Mercury • Different chemical forms – inorganic, metallic, organic ( Hg0 Hg2+ CH3Hg+) • Organic mercury (methylmercury) is the form in fish; bioaccumulates to high levels • Organic mercury from fish is the most significant source of human exposure • Brain and nervous system toxicity – High fetal exposures: mental retardation, seizures, blindness – Low fetal exposures: memory, attention, language disturbances MeHg Consumption Limits US EPA – 0.1 ug/kg-day US FDA – 1 ppm (mg/kg) in tuna Consuming large species such as tuna and swordfish even once a week may be linked to fatigue, headaches, inability to concentrate and hair loss, all symptoms of low-level mercury poisoning. In a study of 123 fish-loving subjects, the researchers found that 89% had blood levels of methylmercury that exceeded the EPA standard by as much as 10 times. How Much Tuna Can You Eat Each Week? A safe level would be approximately 1oz for every 20lb of body weight. So for a 125lb (57kg) person, 1 can of tuna a week maximum. Excitotoxicity-Glutamate Mediated Cell Death Experimental Observations Glutamate induces a delayed cell death in neurons This cell death requires extracellular calcium and is blocked by antagonists of NMDA receptors Hypothesis: Prolonged or inappropriate activation of NMDA receptors underlies glutamate excitotoxicity of neurons Glutamate Synapses Excitatory synapse of brain Required to generate action potentials Both AMPA and NMDA receptors are critical for normal brain function Glutamate synapse NMDA-hi Ca++ permeability Overview of Glutamate and Excitotoxicity Glutamate activates two types of ion channels (AMPA and NMDA) Cell Death is associated with excessive calcium entry through NMDA receptors Both Native and Recombinant NMDA Receptors Can Cause Excitotoxicity Neurons Transfected CHO cells NMDA-induced Excitotoxicity is NR2 Subunit Dependent in Recombinant Expression Systems NMDARS require two NR1 subunits and two NR2 subunits -NR2 family-NR2A, 2B, 2C, 2D -NR2A, NR2B high excitotoxicity potential -NR2C, NR2D lower excitotoxicity potential Calcium and Excitotoxicity Glutamate-mediated apotosis in spinal motor neurons is blocked by calpain inhibitors Expose cells to 10 µM Glu in absence or presence of calpeptin Monitor apoptosis (left panel) or membrane potential (right panel) The Calcium That Triggers Excitotoxicity is Source-Dependent Calcium entry via NMDA receptors can trigger neuronal cell death Calcium entry through other channels (eg. VSCC) does not Location of NMDA receptors is also important, synaptic versus extrasynaptic Calcium Mitochondrial Dysfunction Resulting from Calcium Overload is Source-Specific Mito Vm Synaptic and non-synaptic NMDA Receptors Increase Calcium L-type calcium channel increase calcium Synaptic NMDA receptors and L-type channels do no affect mitochondrial function Extrasynaptic NMDA receptors disrupt mitochondrial function and are linked to excitotoxicity Glutamate Excitoxicity in Oligodendrocytes Historically, oligos were thought to lack NMDA receptors More recent studies demonstrate NMDA and non-NMDA currents in oligos These receptors may be activated by injury or ischemic conditions that result in the release of glutamate Loss of oligo processes may underlie myelin degeneration associated with many diseases such as cerebral palsy, spinal cord injury and multiple sclerosis Glutamate Excitoxicity in Oligodendrocytes Oxygen-glucose deprivation (OGD)model of ischemic damage Leads to loss of oligo processes This is prevented by blockers of NMDA receptors (MK801) Glutamate and Human Brain Trauma Glutamate in Human Brain Following Stroke Glutamate Threonine Glutamate levels remain high after stroke Threonine, a structural amino acid, is measured as a control