* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Programmed Cell Death during Leaf Senescence in Eucommia
Survey
Document related concepts
Gel electrophoresis wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Maurice Wilkins wikipedia , lookup
Molecular evolution wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Non-coding DNA wikipedia , lookup
List of types of proteins wikipedia , lookup
Community fingerprinting wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
DNA vaccination wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Molecular cloning wikipedia , lookup
DNA supercoil wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Transcript
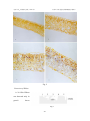
北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 杜仲叶片衰老过程中的细胞程序性死亡 Programmed Cell Death during Leaf Senescence in Eucommia ulmoides Oliv.* 生命科学学院 植物分子与发育学系 98 级 江枫 Abstract Leaf tissues from Eucommia ulmoides Oliv. trees were used to study the mechanism of programmed cell death (PCD) during leaf senescence. Here, we show that DNA ladders were detected by gel electrophoresis only in senescent leaves. DNA fragmentation and nuclear DNA condensation were further confirmed by using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end in situ labeling (TUNEL) method. A 20 kDa DNase was detected only in senescent leaves, which suggested that fragmented DNA would be caused by this DNase. Keywords: DNase―Leaf senescence―Programmed cell death (PCD) ―Eucommia ulmoides Oliv. Cell death is a basic biological process that functions in many aspects of animal and 240 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 plant development and in their responses to stress (Greenberg 1996, Wang et al. 1996, Martins and Earnshaw 1997). Studies on animals have shown that the execution of programmed cell death (PCD) or apoptosis is controlled by a multistep signaling pathway (McConkey and Orrenius 1994, Stewart 1994). Like in animals, it is called as physiological cell death for development and defense (Greenberg 1996), so it may be that any complex multicellular organism will be found cell death a usuful process to have in its repertoire. There are many examples on programmed cell death during plant development, such as senescence of the carpel and petal (Orzáez and Granell 1997a, b), xylogenesis (Mittler and Lam 1995, Fukuda 1996, 1997, Wang and Cui, 1998), aleurone deletion (Wang, M. et al. 1996), the death of the root cap cells (Wang, H. et al. 1996) and somatic embryogenesis (McCabe et al. 1997). Leaf senescence is the final stage of leaf development, which has been thought to be a type of programmed cell death (Noodén and Leopold 1978,Gan and Amasico 1997). The mechanism which controls this process must be genetically regulated by autonomous (internal) factors such as age, reproductive development and phytohormone levels, as well as regulated by environmental signals such as stresses, drought, ozone, nutrient deficiency, pathogen infection, wounding and shading (Gan and Amasico 1997). At the molecular level, till now, more than 30 leaf senescence associated genes (SAGs) have been isolated, cloned and characterized. Most of them encode enzymes that are thought to be involved in cell degeneration and nutrient mobilization, whereas the identity of some SAGs remains unknown (Basanti et al. 1999). Although so much factors and genes regulated leaf senescence were observed, it was unclear whether leaf senescence shared any biochemical or genetic pathways with other types of PCD in plants or animals. Yen and Yang (1998) have reported the * Foundation items: Jun Zheng Fudation of Peking University 241 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 detection of DNA fragmentation in naturally senescent leaves from five plant species, which proved that senescence of the leaves must share some similar pathways observed in other types of PCD in both plant and animals. In this paper, DNA ladders and DNA fragmentation were also detected in naturally senescence leaves from Eucommia ulmoides Oliv. Trees. In addition, we have detected DNase during leaf senescence, providing important information on the mechanism of leaf senescence. Materials and Methods Plant materials Leaves at different developmental stages, from young (full expansion), green1 (May to Aug.), green2 (Sep. to Nov.), yellow and dry leaves were obtained from strong and healthy Eucommia ulmoides Oliv. trees which were grown on the campus of Peking University, Beijing, China. DNA extraction and analysis Genomic DNA was isolated from leaf tissues using a CTAB method. Leaf tissues were ground in liquid N2 immediately after being collected from the plants and then the frozen samples were homogenized in an extraction buffer that contained 2% CTAB, 100 mmol/L Tris-HCl, pH 8.0, 20 mmol EDTA, pH 8.0 and 1.4 mmol NaCl, and the mix incubated at 65℃ for 1 h. After this, Equal volumes chloroform/isoamyl alcohol (24/1) were added and the tubes inverted 30 min and the samples centrifuged at 12,000 g for 10 min, repeated twice. The DNA was precipitated by adding 2/3 vol of isopropyl alcohol at -20℃ for 30 min. DNA samples were digested with DNA-free RNase for 1 h at 37℃ and the DNA content determined. For DNA analysis, samples 242 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 of 4 μ g DNA were loaded per lane and run on a 1% agarose gel at constant 70 V. The DNA was visualized by staining with 0.5 μg /ml ethidium bromide. TUNEL assay Leaf tissues were cut and fixed in 10% formalin with PBS buffer pH 7.2 overnight. The samples were dehydrated through a graded series of ethanol and embedded in paraffin. Sections of 8-10μm thickness were cut using a microtome type 860. After dewaxing and rehydration, sections were incubated with proteinase K (20μg/ml) for 15 min at 37℃ and rinsed twice with PBS. Then sections were stained for PCD observation with in situ cell death detection kit, AP (Boehringer, Mannheim, Germany). Reaction products were dark blue. In positive control, DNase I (grade I, 0.5 mg/ml in 50 mmol/L Tris-HCl, pH 7.5, 1 mg/ml BSA) was added before TUNEL reaction mixture for 10 min at room temperature to induce DNA strand breaks. In negative control, terminal transferase was omitted. Extraction of protein from leaf tissues. For extraction of the soluble protein, leaves were thoroughly ground in liquid N2 in a prechilled mortar, and then the powder was suspended in an extraction buffer that contained 0.5 mol/L Tris-HCl, pH 6.8, 5% 2-mercaptoethanol and 10% glycerol, 2.5% SDS and a trace of bromophenol blue. After centrifugation at 15,000 g for 15 mins at 4 ℃. The pellets were further ground and washed three times with the same buffer. All the supermatants were combined together as the extract of soluble protein. 243 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 Detection of molecular species of nucleases using DNA-SDS polyacrylamide gel electrophoresis Nucleases with their molecular weights were detected by DNA-SDS polyacrylamide gel electrophoresis, as described previously (Sodmergen and S. Kawano 1991). Separation gel contained 50% acrylamide (Promega), 1.25% bisacrylamide (Promega), 2 mg/ml salmon sperm DNA (Sigma), 0.4% SDS (Sigma), 1.5 mol/L Tris·HCl, pH 8.8, 0.5% ammonium persulfate, and 0.1% (v/v) TEMED. Spacer gel contained 12% acrylamide, 0.4% bisacrylamide, 0.4% SDS, 0.5 mol/L Tris·HCl, pH6.8, 0.5% (w/v) ammonium persulfate, and 0.1% (v/v) TEMED. Electrophoresis was carried out at constant 20 mA at room temperature. Total time was about 3-4 hours. After electrophoresis, the protein standard marker was cut off and stained with 0.25% coomassie blue. The left gels were washed to remove SDS for 1h in the washing buffer (10 mmol/L Tris-HCl,pH 8.5, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 0.1% mercaptoethanol and 0.1 mmol/L PMSF).Then shaking in the washing buffer overnight. The next day, after being washed for 1 h, the gel was incubated for enzymatic activity in the washing buffer contained 10 mmol/L CaCl2 and 10 mmol/L MgCl2 at 37℃ overnight. Then the gel was stained with ethidium bromide for 30 mins. DNase activity was visualized under UV light. Results Detection of DNA ladder during leaf senescence 244 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 The DNA from leaves at different development stages was extracted and analysed in agarose gels. The results indicate that DNA from young and green1 leaves shows no degradation (Fig.1, lane1 and lane2). However, as we proceed from green2 to yellow leaves, bands of DNA degradation can be observed clearer (Fig.1, lane3 and lane4). The size of the DNA bands corresponds to multiples of around 200bp, suggesting that they are produced by internucleosomal degradation of the DNA. In the Fig. 1 dried leaf, the DNA became a smear and the laddering was reduced to become unvisualized in the agarose gel (Fig.1, lane5). DNA fragmentation analysis The DNA fractionation was studied at the cellular level in thin sections of non-senescent, senescent leaves using the TUNEL reaction. Although the TUNEL reaction is not exclusive for DNA degradation occurring at the internucleosomal region, it is a very useful technique to detect in situ those nuclei in which the number of DNA ends has increased as a consequence of DNA degradation. In Figure2, the nuclei of mesophyll cells in green2 and yellow leaves were labeled by TUNEL (Fig.2, C and D) and green2 leaves were labeled stronger than yellow leaves. By contrast, there were no TUNEL staining in young and green1 leaves, as shown in Fig.2, A and B, indicating the nuclei of the cells in these leaves remain intact. 240 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 Fig. 2 Detection of DNase A 20 kDa DNase was detected only in green2 leaves Fig.3 241 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 (Fig.3,Lane3), which suggests that fragmented DNA may be caused by this DNase. No DNase was detected in other developmental stages leaves (Fig.3, Lane1, 2, 4 and 5). Summary of the experiments (Table 1) DNA ladders and TUNEL Label were detected only in senescence leaves (green2 and yellow); The degradation of PARP localized around the nuclei and appears before emergence of DNase; A 20 kDa DNase was detected only in green2 leaves which began to senescence. Table 1 Results of the study Young Green1 Green2 Yellow Dry DNA ladders No No Yes Yes Smear TUNEL assay DNase No No Yes Yes Not done No No Yes(20kDa) No No Discussion The relationship between PCD and leaf senescence Although the leaf senescence has been studied for many years, most of the studies focussed on the breakdown of the chloroplast and the changing of proteinase activity (Thomson and Platt-Aloia 1987). It is interesting to note that only a few reports give DNA damage or alteration at the nuclear levels an important role in leaf senescence (Yen and Yang 1998). This was possibly because in most cases senescence had been studied in leaves where the chloroplast was the first organelle showing the effects of senescence, and the effects on the nuclei occurred rather late (Orzáez and Granell 242 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 1997a). Bleecker and Patterson (1997) has reported that senescence and abscission of leaves are PCD processes. And Orzáez and Granell (1997a, b) indicated that senescence of the carpel and petal were also PCD processes. This study indicated that at the cellular level, senescence of the leaves takes place in a similar manner as that observed in animals by producing condensed nuclei and yielding fragmented DNA, a 20 kDa DNase, DNA ladders during leaves senescence, and caspase-3-like protease(s) in early stage of leaf development. These further demonstrated that leaf senescence was a PCD progress. And this investigation also found that DNA fragmentation was detected not only in yellow leaves but also in green2 leaves. This suggested that the signals triggered natural leaf senescence should occur early, before the leaf began to show the sign of yellowing in which cells have already entered the third stage of PCD. Since most genes associated with leaf senescence expressed during the latter phases of senescence, it is necessary to further identify the genes involved in the initial step of this process, which should lead to a deeper understanding of its mechanism (Yen and Yang 1998). But above these suggested that the differentiation of leaf cells should be last stage of cell differentiation (Cui 1997), the differentiation process of these cells could be PCD. Once all cells in leaf entered the last stage of PCD, the leaf should appear the characters of senescence. Mechanism for PCD in leaf senescence According to the investigation on animal and medical, PCD comprises of three stages: initiation, effector and degradation, during which the caspase family plays the key role. During the third stage of PCD, specific endonucleases attack nuclear DNA in the internucleosomal linker regions between nucleosomal cores, resulting the 243 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 production of double-stranded, low molecular weight oligonucleosomal DNA fragments about 180 to 200 bp in size (Cohen 1993). Perez et al. (2000) identified BFN1, a bifunctional nuclease induced during leaf and stem senescence in Arabidopsis, which would be an excellent tool with which to study the mechanisms of the senescence induction. Our experiments also showed that during leaf senescence, a 20 kDa DNase was detected only in green2 leaves (Sep. to Nov.), the stage that DNA ladders and fragments began to emergence and were strongly detected, which suggested that fragmented DNA may be caused by this DNase. In an elegant series of experiments, the groups of Wang and Nagata showed that the DNA ladder nuclease (now known as caspase-activited DNase, or CAD) pre-exists in living cells as an inactive complex with an inhibitory subunit, dubbed ICAD (Nagata 2000). Activation of CAD occurs by means of caspase-3-mediated cleavage of the inhibitory subunit, resulting in the release and activation of the catalytic subunit (Liu and Zou et al., 1997; Enari et al., 1998; Sakahira et al., 1998). So PCD signaling emerged very early during leaf development, perhaps at the stage of budding or earlier. These suggested that main process of PCD in plants could be the same as in animals, but there could be different death sign and the direction in which produce from PCD having gone between plants and animals. The study provides important findings in the study of the mechanism of PCD in plants. Acknowledgement I would like to thank Jun Zheng Foundation for providing me this chance to do the scientific research, and Cao Jing for sharing of unpublished observations, and my supervisor Prof. Cui Keming for his instruction on scientific attitude and method and 244 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 his critical reading of this manuscript. I am also grateful to Prof. Sodmergen for giving his advice on technology, and other teachers and colleagues in our laboratory for their help. References Basanti, B. and Biswal-U-C 1999. Leaf senescence: Physiology and molecular Biology. Current-Science-Bangalore. Sept., 77(6): 775-782. Bate, N.J., Rothstein, S.J. and Thompson, J.E. 1990. Expression of nuclear and chloroplast photosynthesis-specific genes during leaf senescence. J. Exp. Bot. 239: 801-811. Bleecker, A.B. and Patterson, S.E. 1997. Last exit: senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell. 9: 1169-1179. Cohen, J.J. 1993. Apoptosis. Immunol. Today 14: 126-130. Cui K.-M. 1997. Switch and stages of plant cell differentiation. Life Sci. 9: 1147-1156 Enari, M. et al. 1998. A caspase–activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43-50. Fukuda, H. 1996 Xylogenesis: initiation, progression, and cell death. Annu. Rev. Plant Mol. Biol. 47: 299-325. Fukuda, H. 1997. Tracheary element differentiation. Plant Cell 9: 1147-1156. Gan, S. and Amasino, R.M. 1997. Making sense of senescence. Molecular genetic regulation and manipulation of leaf senescence. Plant Physiol. 113: 313-319. Greenberg, J.T. 1996. Programmed cell death: A way of life for plants. Proc. Natl. Acad. Sci. USA 93: 12094-12097. Liu, X., Zou, H. Slaughter, C. and Wang, X. DFF, 1997. A heterodimeric protein 245 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175-184. Martins, L.M. and Earnshaw, W.C. 1997. Apoptosis: Alive and kicking in 1997. Trends Cell Biol. 7: 111-114. McCabe, P.F., Levine, A., Meijier, P.J., Tapon, N.A. and Pennell, R.I. 1997. A programmed cell death pathway activated in carrot cell cultured at low cell density. Plant J. 12: 267-280. McConkey, D.J. and Orrenius, S. 1994. Signal transduction pathways to apoptosis. Trends Cell Biol. 4: 370-375. Mittler, R. and Lam, E. 1995. In situ detection of nDNA fragmentation during the differentiation of tracheary elements in higher plants. Plant Physiol. 108: 489-493. Nagata, S. 2000. Apoptic DNA fragmentation. Exp. Cell Res. 256: 12-18. Noodén, L.D. and Leopold, A.C. 1978. Phytohormones and the endogenous regulation of senescence and abscission. In D Letham Goodwin, T Higgins, eds, Phytohormones and Related Compounds: A comprehensive Treatise, Vol. 2. Elsevier, New York, pp 329-369. Orzáez, D. and Granell, A. 1997a. DNA fragmentation is regulated by ethylene during carpel senescence in Pisum sativum. Plant J. 11: 137-144. Orzáez, D. and Granell, A. 1997b. The plant homologue of the defender against apoptotic death gene is down-regulated during senescence of flower petals. FEBS Lett. 404: 275-278. Perez, A.M.A., Abler, M.L., De, R.E.J. et al. 2000. Identification of BEN1, a bifunctional nuclease induced during leaf and stem senescence in Arabidopsis. 246 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 Plant Physiology Rockville. 122 (1): 169-179. Sakahira, H., Enari, M. & Nagata, S. 1998. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 391: 96-99. Stewart, B.W. 1994. Mechanism of apoptosis: Integration of genetic, biochemical, and cellular indicators. J. Natl. Cancer Inst. 86: 1286-1296. Sodmergen, Kawano, S. et al. 1991. Degradation of chloroplast DNA in second leaves of rice (Oryza sativa) before leaf yellowing. Protoplasma. 160: 89-98. Taylor, C.B., Bariola, P.A., Delcardayre, S.B., Raines, R.T. and Green, P.J. 1993. RNS2: a senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proc. Natl. Acad. Sci. USA 90: 5118-5122. Thomson, W.W. and Platt-Aloia, K.A. 1987. Ultrastructure and senescence in plants. In W Thomson, E Nothnagel, R Huffaker, eds, Plant Senescence: Its Biochemistry and Physiology. American Society of Plant Physiologists, Rockville, MD, pp. 20-30. Wang, H., Li, J., Bostock, R.M. and Gilchrist, D.G. 1996. Apoptosis: A functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development . Plant Cell 8: 375-391. Wang, M., Oppedijk, B.J., Lu, X., Van Duijin, B. and Schilperoort, R.A. 1996. Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Mol. Biol. 32: 1125-1134. Wang, Y.-Q. and Cui, K.-M. 1998. Programmed cell death during the vessel element differentiation of the secondary xylem in Eucommia Ulmoides shoots. Acta Bot. Sin. 40 (12): 1102-1107. Yen, C.H. and Yang, C.H. 1998. Evidence for programmed cell death during leaf 247 北京大学 政学者论文集(2001 年) 杜仲叶片衰老过程中的细胞程序性死亡 senescence in plants. Plant Cell Physiol. 39: 922-927. 作者简介: 江枫,女,1981 年生于福建省福州市,中学就读于福州市第八中学,因学 习成绩优异,被校批准跳级一年,直接参加高三学习。1997 年曾获全国高中数 学联赛一等奖,并获得免试保送北京大学数学学院的机会。但因对生物学有着浓 厚的兴趣,1998 年考入北京大学生命科学学院,就读于植物分子与发育学系。 2000 年获得北京大学学习优秀奖。大学三年学习成绩名列专业第一名,并准备 申请自费留学美国攻读博士学位。 感悟与寄语: 转眼间在北大已经过去了三年,我改掉了自小以来的以学习为中心的生活方 式,明白了生活的灿烂是学习远远不能给予的。既然选择了生物,今后大部分的 时间和精力必然会投入于研究工作中,我很早就期待着能有机会体验一下科学家 的生活。很感谢“君政基金”给予了我一个参与科学研究的机会,尝到了它的酸 甜苦辣,更加对自己的理想——作一名科学家坚定不移。我们实验室的工作是研 究正常发育状态下的树木的细胞程序化死亡,由于树木的生长周期长,与其他的 实验室相比,实验周期长,技术难度大,在国际上也比较少人涉足这个领域,选 择这样的课题,足见导师不急功近利,一心钻研的科学精神。一年时间对于研究 工作是远远不够的,我没有完成发表一篇论文的任务,但是对于研究我有了许多 创新性的见解,这对我在将来的工作是大有裨益的。希望所有向往科学的勇者都 “有志者事竟成”! 指导教师简介: 248 北京大学 政学者论文集(2001 年) 崔克明 杜仲叶片衰老过程中的细胞程序性死亡 教授,男,1941 年 2 月生,山东寿光人。1966 年毕业于北京大学 生物学系植物学专业。现任北京大学生命科学学院教授,植物学专业博士生导师, 植物分子及发育生物学系副主任,兼任中国植物学会结构及生殖生物学专业委员 副主任、中国杜仲综合开发协会常务理事、中国林学会杜仲研究会常务副主任委 员、北京植物学会副理事长、国家自然科学基金委员会植物学学科评审组成员、 国家科技奖励评委会委员、北京市学位委员会学科评审组成员和《植物学报》编 委等职。 249