* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Energy: Conservation and Interconversion Demonstration:
Survey
Document related concepts
Copper in heat exchangers wikipedia , lookup
Heat capacity wikipedia , lookup
Heat equation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal conduction wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Thermodynamic system wikipedia , lookup
Conservation of energy wikipedia , lookup
Heat transfer physics wikipedia , lookup
Gibbs free energy wikipedia , lookup
Adiabatic process wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Transcript
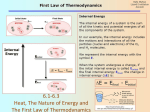
1 Energy: Conservation and Interconversion The energy of the Universe is constant, but can be converted from one form to another We will be concerned with these three forms of energy: • • • Chemical energy Electrical energy Nuclear energy The energy liberated in these processes can be in the form of light, charge or current, and heat. Nuclear energy brings into focus the fact that energy and mass can be interconverted. Although the interconversion of energy and mass is manifested most clearly in nuclear processes, it is true for all processes. This idea places mass determination at the center of energy conversion. Interestingly, concepts of mass measurement were used in the 18th century by Dalton, Avogadro, and Cannizzaro to formulate the Atomic Theory of Matter. Even now, mass and its origin are at the center of the “big” unanswered questions about the Universe that is sometimes called the “theory of everything”. Demonstration: The demonstration on the oxides of nitrogen illustrates a number of important concepts: At room T: NO + ½ O2 → NO2 Color change denotes spontaneous reaction. Flask gets warm. The reaction evolves heat. The origin of color is something we’ll discuss when we talk about chemical bonding. The electron distribution around nitrogen changes. At dry ice/acetone T: NO2 + NO → N2O3 (blue liquid) N2O3 + ½ O2 → N2O4 (colorless gas) Observe temperature dependence: 2NO2 = N2O4 At higher temperatures, the gas mixture attains a strong red-orange color. The color fades at lower temperature. Qualitatively, we know that the reaction as written liberates heat. LeChatelier’s Principle tells us that to compensate for increased T, the reaction should run in the direction to absorb the added heat. That is, the reaction runs from right to left. 2 Two important postulates of thermodynamics will guide our thinking The First Law is a statement of energy conservation, and provides guidelines for us on what physical processes can happen The Second Law is more restrictive, telling us that energy conservation alone is not a sufficient condition on whether a process occurs or not. The Second Law tells us what processes actually do happen. Concepts of Heat and Work Recall that 1 Joule is the amount of energy required to raise the temperature of 1.00 grams of liquid water exactly 0.239 °C. A more commonly used unit of heat is the calorie, the amount of heat required to raise the temperature of 1.00 grams of liquid water by 1.00 °C. The calorie is clearly a unit of heat, while the Joule, with units of kg (m/sec)2, is obviously a mechanical unit. Nevertheless, we can write the equivalence 1 cal = 4.184 Joule – The Mechanical Equivalent of Heat We like to think of heat as a highly degraded form of energy, while work is a more organized form of energy. A device that converts heat into work is therefore a valuable energy conversion device. The internal combustion engine is such a device. The efficiency of this conversion is clearly an important topic – we’ll return to this soon. Heat, Work, and Energy To see the distinctions among these terms, let’s divide the universe into the System (the part we focus on) and the Surroundings (everything else). We can change the energy of the system by Transferring heat from the surroundings to the system – heat with a Bunsen burner. Here, the chemical reaction, the combustion of methane, occurs in the surroundings, transfers its heat to the system. Having the surroundings do work on the system – compress the system, transfer electrical charge (battery charger) 3 The diagrams below help us understand how energy, heat, and work are related. Note that heat and work are processes or interactions between the system and the surroundings. Heat and work depend on the way the process is carried out. They “depend on the path”. Note that positive heat absorbed by the system results in an increase in the internal energy of the system. Similarly, the surroundings can do work on the system and increase the energy of the system . ∆E WORK HEAT Important distinction: Internal energy, E, is an intrinsic property of the system. Heat and work are processes, not intrinsic quantities. Similarly, when negative heat is absorbed by the system, or negative work is done on the system, the internal energy decreases. ∆E WORK HEAT These diagrams help us focus on the fact that two processes that involve interactions between system and surrounding determine a physical property of the system. The First Law of Thermodynamics says that ∆E = q + w Significance: q and w are processes that depend on path, but their sum is independent of path E is a thermodynamic function of state (of the system). So are variables like T, P, V. 4 Functions of state vs. processes Drive from Rochester to Denver: Path 1: Take I-90 west to Chicago, through Wisconsin, Minnesota, South Dakota, Wyoming, then I-25 south to Denver. Path 2: Take I-90 East to NYC, take I-95 south through New Jersey, Delmarva Peninsula to Jacksonville, Florida. Take I-10 west along the Gulf Coast (wave to my Uncle in Gulfport, who is still living in a FEMA trailer), catch I-25 North in Arizona. Heat absorbed: measured by gasoline consumption Work done: measured by tire wear These will be different for the two paths. What is independent of path is the Change in elevation: ~ 5000 ft Change in temperature: ?? Change in barometric pressure: ?? These are functions of state – they just depend on the characteristics of the environments of Rochester and Denver. 5 Pressure-Volume Work Let’s consider a simple example of the work done on the system (considered to be a gas) when a constant external pressure is applied to it. Work done on the system = -(external force × displacement) External force = external pressure × surface area W = -P × A × ∆h (note the minus sign) A × ∆h = ∆V = Vfinal - Vinitial W = -P∆V = -P(Vfinal - Vinitial) Let’s do a couple of sample problems: Apply an external pressure of 1.0 atmospheres as a gas expands from 0.5 L to 1.0 L. W = -1.0 atm (1.0 L – 0.5 L) = -0.5 L-atm. Not a particularly useful unit. Since pressure is a force per unit area, the appropriate mechanical SI unit for pressure is N/m2. Turns out that atmospheric pressure is 1.013 × 105 N/m2. Remember that 1 liter = 1000 cm3 = 10-3 m3. (Do you know why?). Therefore 1 L-atm = 1.013 × 105 N/m2 × 103 m3 = 101.3 N-m = 101.3 J This is an important unit conversion. 101.3J =-50.6 J. Note that negative work is done on the 1L − atm system. Note also for a compression, the final volume is smaller than the initial volume. So, the work of compression on the system is positive. For the expansion W = -0.5 L-atm ×