* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Coenzyme A and Acyl Carrier Protein
Artificial gene synthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Point mutation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Lipid signaling wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Butyric acid wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
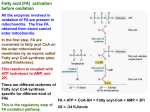
Coenzyme A and acyl carrier protein: structure, occurrence, biology and analysis. Coenzyme A and Acyl Carrier Protein STRUCTURE, OCCURRENCE, BIOLOGY AND ANALYSIS Before a fatty acid can be metabolized in tissues, for example by being esterified, oxidized or subjected to synthetic modification, it must usually be activated by conversion to a Coenzyme A ester or acyl-CoA, with the fatty acid group linked to the terminal thiol moiety. This is true for the most primitive organisms, such as Archaea, through to humans (with the exception only of certain bacteria which utilize fatty acyl phosphates). A thiol ester is a highly energetic bond that permits a facile transfer of the acyl group to receptor molecules, whether it is the simplest fatty acid of all, acetic acid (i.e. as acetyl-CoA), or one of the long-chain fatty acids. a N HC NH2 C N β-mercaptoethylamine β-alanine C N C O CH N CH2 O O CH3 O P O P O CH2 _ _ O O C CHOH C NH CH2 CH2 C NH CH2 O CH3 CH2 S X O 4-phosphopantothenic acid OH O _ PO3 4-phosphopantetheine CoA X = H acyl-CoA X = OCR adenosine 3',5'-diphosphate a Coenzyme A (CoASH or CoA) itself is a complex and highly polar molecule, consisting of adenosine 3’,5’-diphosphate linked to 4-phosphopantethenic acid (vitamin B5) and thence to β-mercaptoethylamine, which is directly involved in acyl transfer reactions. The adenosine 3’,5’-diphosphate moiety functions as a recognition site, increasing the affinity of CoA binding to enzymes. While acyl-dephospho-CoAs lacking the 3’-phosphate group on the ribose moiety have been detected in tissues, their function is unknown. Not only is CoA intimately associated with most reactions of fatty acids, but it is also a key molecule in the catabolism of carbohydrates via the citric acid cycle in which acetyl-CoA is a major end-product. The genes encoding the enzymes for coenzyme A biosynthesis have been identified and the structures of many proteins in the pathway have been determined. Although there are substantial sequence differences between prokaryotes and eukaryotes, coenzyme A is assembled in five steps from pantothenic acid in essentially the same way in both groups. However, pantothenic acid per se can only be synthesised by microorganisms (including gut flora) and plants and must be acquired largely from the diet by animals. In animals, the process is believed to occur entirely in the cytosol of cells, and the first and rate-limiting step involves the enzyme pantothenate kinase, several isoforms of which are known. It is interesting that the 4-phosphopantetheine moiety, linked via its phosphate group to the hydroxyl group of serine, is the active component in another important molecule in lipid metabolism, Acyl Carrier Protein (ACP). This is a small (8.8 kDa) but ubiquitous and highly conserved carrier of acyl groups during the synthesis of fatty acids. In yeast and mammals, it forms a separate region within a multifunctional fatty acid synthase complex, but in bacteria and plastids it remains as a small monomeric protein, though closely associated with the other elements of the fatty acid synthase (see our web page on saturated fatty acids). The phosphopantetheine moiety in effect provides a long flexible chain, which permits the intermediates to remain covalently linked to the synthases in an energy-rich linkage with access to spatially distinct enzyme-active sites in a manner that resembles an assembly line. However, W.W. Christie © lipidlibrary.aocs.org 1 Coenzyme A and acyl carrier protein: structure, occurrence, biology and analysis. the final step in fatty acid synthesis in many types of organism is transfer of the fatty acyl group from ACP to CoA. Intracellular free fatty acids arising from synthesis de novo or from the diet must be activated by a fatty acyl-CoA synthetase (fatty acid:CoA ligase) before they can be utilized for the synthesis of triacylglycerols, wax esters, long-chain aldehydes and alcohols, and complex lipids, or for covalent modification of proteins by myristoylation or palmitoylation. In addition, the fatty acyl-CoA synthetases are central to many aspects of intermediary metabolism. Acyl CoA synthetases activate fatty acids through a process that is energy–dependent and requires ATP and CoA. It is a two-stage process, requiring magnesium ions in the first step, which involves the formation of an acyl-AMP intermediate. ATP is consumed and AMP and pyrophosphate are produced. a ATP + fatty acid Acyl-AMP + CoA Acyl-AMP + PPi Acyl-CoA + AMPa At least five families of acyl CoA synthetases are known in humans with specificities for fatty acids in different chain-length groups, i.e. short-chain (2-3 carbons), medium-chain (4-12 carbons), long-chain (12-22 carbons), so-called ‘bubble-gum’ (14-24 carbons) and very-long-chain (18-26 or more carbons) fatty acid substrates. The enzymes are distinguished by two highly conserved sequence elements, i.e. an ATP/AMP binding motif, which is common to enzymes that form an adenylated intermediate, and a fatty acid binding motif. Multiple isoforms of these enzymes are known to be present in animals and other life forms, and six have been identified in the yeast genome while there are at least 26 in the human genome, for example. They are generally believed to be membrane bound and each isoform appears to be located in at a unique subcellular location, where it may contribute acyl-CoA to different metabolic pools or where it can participate in the transport of fatty acyl moieties across membranes. For example, there is appreciable sequence homology between the very-long-chain acyl CoA synthetases and certain fatty acid transport proteins in animals, and the significance of this is under active investigation. Acetyl-CoA derived via the citric acid cycle or from acetate via a CoA synthetase is of course the primary precursor for fatty acid sythases (see our web page on saturated fatty acids). In addition, short-chain acyl-CoAs including free CoA, acetyl-CoA and malonyl-CoA are well known regulators of metabolic flux, with the ratio of acetyl-CoA to free CoA tightly regulating glycolysis and fatty acid oxidation. As well as its role in fatty acid synthesis, malonyl-CoA reduces fatty acid oxidation by inhibiting the transport of acylCoA into mitochondria. In addition to their role in lipid biosynthesis and catabolism, CoA esters have been shown to regulate the activities of a variety of enzymes, including that of acetyl-CoA carboxylase, an essential enzyme in fatty acid synthesis. Many genes and enzymes are regulated by deacylation and acylation via various short-chain acyl-CoAs, such as acetyl- and succinyl-CoA. Long-chain acyl-CoA esters also bind to certain hormone receptors and have a signalling function. Many of the effects observed for free fatty acids in nuclear signalling may also be attributable to acyl-CoA esters. However, this would take us into another very substantial realm of biochemistry. Many bacterial species, both Gram-negative and Gram-positive, synthesise long-chain acyl-CoA esters for lipid synthesis, and this enables them to make efficient use of exogenous fatty acids. However, other bacterial species do not make use of CoA in this way but instead utilize newly synthesised acyl groups linked via the thiol bond to the acyl carrier protein (ACP), i.e. in the form that they are produced by the type II fatty acid synthase. Some species, including Escherichia coli, use both acyl-CoA esters and acylACPs for synthesis of phosphatidic acid de novo. Many other bacterial species activate fatty acids in a very different way, i.e. as the fatty acyl phosphates (see below). CoA esters are required for a number of processes in addition to esterification. During fasting or starvation, intracellular long-chain fatty acids mobilized from adipose tissue reserves are catabolized as fuel by the mitochondrial β-oxidation pathway, and they must be first be converted to the CoA esters W.W. Christie © lipidlibrary.aocs.org 2 Coenzyme A and acyl carrier protein: structure, occurrence, biology and analysis. prior to synthesis of carnitine derivatives for translocation into the mitochondrion. Medium-chain fatty acids can enter mitochondria without carnitine transport but they must be still activated before βoxidation can occur. Similarly, peroxisomes in animal cells have a distinct fatty acid β-oxidation system with a separate set of enzymes, including as many as three acyl-CoA oxidases. The acyl-CoA oxidase 1 catalyses the βoxidation of straight-chain acyl-CoAs, while acyl-CoA oxidase 2 is involved in the oxidation of the sidechain of bile-acid precursors, and acyl-CoA oxidase 3 catalyses the oxidation of methyl branched-chain CoA esters. Activation is needed also for α-oxidation in tissues. In addition, most other biological reactions of fatty acids including chain elongation and desaturation (plants are an exception) require their activation. As they have both polar and hydrophobic molecular components, CoA esters of long-chain fatty acids have strong detergent-like physical properties and have the potential to be disruptive towards cells. The intracellular concentration of free acyl-CoA esters is tightly controlled by feedback inhibition of the acylCoA synthetase, and is buffered by specific acyl-CoA binding proteins in the cytoplasm, which in effect reduce the concentration by up to 104 fold. These are discussed in relation to plants elsewhere on this website. Mitochondrial acyl-CoA concentrations are 10 fold higher than in the cytoplasm. At high concentrations, acyl-CoA are non-specific inhibitors of innumerable enzyme systems, and they must be removed from cells in part as their acyl-carnitine derivatives (see the appropriate web page). Catabolism: There is a family of acyl-CoA thioesterases, located in most cellular compartments, which catalyse the hydrolysis of acyl-CoAs to the free fatty acid and coenzyme A. Suggested functions for these enzymes include ligand supply for nuclear receptors, regulation and termination of fatty acid oxidation in mitochondria and peroxisomes, and control of the supply of acetate and of coenzyme A. Pathological conditions, including certain hereditary conditions, that lead to impaired metabolism and accumulation of CoA and acyl-CoA within cells, trigger a sequence of reactions, which give rise to chronic illnesses. Alternatives to CoA Esters – Fatty Acid Phosphates Most bacteria, including such important human Gram-positive pathogens as Streptococcus pneumoniae and Staphylococcus aureus, are now known to lack the glycerophosphate acyl transferase enzymes that make use of CoA. Instead, a fatty acyl phosphate is the reactive acyl donor. a R O S _ ACP + PO4 O HO R O P O + ACP _ O a The acyl phosphate is produced by reaction of acyl-ACP with phosphate catalysed by an acylACP:phosphate acyltransferase, and the product then requires specific acyltransferases so that it can be utilized for the synthesis of phosphatidic acid. Although acyl phosphates are less stable than thio esters in vitro, this is obviously not a problem in vivo. Analysis of Coenzyme A Esters The profile of CoA esters in tissues can be an important indication of metabolic status, but because of their strongly amphipathic character, analysis is fraught with technical difficulties. The procedures cited below are typical of those in use (we have no personal experience). Extraction from tissues presents problems, and it may even be necessary to add a specific binding protein to ensure quantitative W.W. Christie © lipidlibrary.aocs.org 3 Coenzyme A and acyl carrier protein: structure, occurrence, biology and analysis. recoveries. Having obtained an appropriate extract, methods are available to separate short- and longerchain fractions, and individual components can then be resolved by reversed-phase high-performance liquid chromatography. However, quantification can be a further problem, as it is not a straightforward matter to produce true solutions of pure compounds as standards. Electrospray-ionization tandem mass spectrometry now appears to hold particular promise for analysis. Recommended Reading Byers, D.M. and Gong, H. Acyl carrier protein: structure-function relationships in a conserved multifunctional protein family. Biochem. Cell Biol., 85, 649-662 (2007). Færgeman, N.J. and Knudsen, J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem. J., 323, 1-12 (1997). Kirkby, B., Roman, N., Kobe, B., Kellie, K. and Forwood, J.D. Functional and structural properties of mammalian acyl-coenzyme A thioesterases. Prog. Lipid Res., 49, 366-377 (2010). Knudsen, J., Neergaard, T.B., Gaigg, B., Jensen, M.V. and Hansen, J.K. Role of acyl-CoA binding protein in acyl-CoA metabolism and acyl-CoA-mediated cell signaling. J. Nutr., 130, 294S-298S (2000). Leonardi, R., Zhang, Y.-M., Rock, C.O. and Jackowski, S. Coenzyme A: back in action. Prog. Lipid Res., 44, 125-153 (2005). Li, Q., Zhang, S., Berthiaume, J.M., Simons, B. and Zhang, G.-F. Novel approach in LC-MS/MS using MRM to generate a full profile of acyl-CoAs: discovery of acyl-dephospho-CoAs. J. Lipid Res., 55, 592-602 (2014). Magnes, C., Suppan, M., Pieber, T.R., Moustafa, T., Trauner, M., Haemmerle, G. and Sinner, F.M. Validated comprehensive analytical method for quantification of coenzyme A activated compounds in biological tissues by online solid-phase extraction LC/MS/MS. Anal. Chem., 80, 5736-5742 (2008). Mitchell, G.A., Gauthier, N., Lesimple, A., Wang, S.P., Mamer, O. and Qureshi, I. Hereditary and acquired diseases of acyl-coenzyme A metabolism. Mol. Genetics Metab., 94, 4-15 (2008). Soupene, E. and Kuypers, F.A. Mammalian long-chain acyl-CoA synthetases. Exp. Biol. Med., 233, 507521 (2008). Tong, F.M., Black, P.N., Coleman, R.A. and DiRusso, C.C. Fatty acid transport by vectorial acylation in mammals: Roles played by different isoforms of rat long-chain acyl-CoA synthetases. Arch. Biochem. Biophys., 447, 46-52 (2006). Watkins, P.A. Very long-chain acyl-CoA synthetases. J. Biol. Chem., 283, 1773-1777 (2008). Zhang, Y.-M and Rock, C.O. Acyltransferases in bacterial glycerophospholipid synthesis. J. Lipid Res., 49, 1867-1874 (2008). William W. Christie James Hutton Institute (and Mylnefield Lipid Analysis), Invergowrie, Dundee (DD2 5DA), Scotland th Last updated: April 8 , 2014 W.W. Christie © lipidlibrary.aocs.org 4