* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CHAPTER 3: The Experimental Basis of Quantum
Eigenstate thermalization hypothesis wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Renormalization wikipedia , lookup
Future Circular Collider wikipedia , lookup
ATLAS experiment wikipedia , lookup
ALICE experiment wikipedia , lookup
Wheeler's delayed choice experiment wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Elementary particle wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Photon polarization wikipedia , lookup
Photoelectric effect wikipedia , lookup
Double-slit experiment wikipedia , lookup
Electron scattering wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
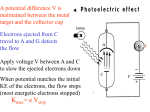
CHAPTER 3 Prelude to Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X-Ray and the Electron Determination of Electron Charge Line Spectra Quantization Blackbody Radiation Photoelectric Effect X-Ray Production Compton Effect Pair Production and Annihilation Max Karl Ernst Ludwig Planck (1858-1947) Problems due Wednesday Sept. 23rd: Chapter 3: problems: 7, 9, 14, 15, 16, 18, 19, 24, 25, 26 X-Ray Production: Theory An energetic electron passing through matter will radiate photons and lose kinetic energy, called bremsstrahlung. Since momentum is conserved, the nucleus absorbs very little energy, and it can be ignored. The final energy of the electron is determined from the conservation of energy to be: E f Ei h Ei Ef h Photons also have momentum! Use our expression for the relativistic energy to find the momentum of a photon, which has no mass: E (mc ) p c 2 2 2 Alternatively: When radiation pressure is important: 2 2 E h h p c c h 2 p k 2 Comet tails (other forces are small) Viking spacecraft (would've missed Mars by 15,000 km) Stellar interiors (resists gravity) X-Ray Production: Experiment Current passing through a filament produces copious numbers of electrons by thermionic emission. If one focuses these electrons by a cathode structure into a beam and accelerates them by potential differences of thousands of volts until they impinge on a metal anode surface, they produce x rays by bremsstrahlung as they stop in the anode material. Compton Effect Ep, pp Photons have energy and momentum: E hc / p h/ When a photon enters matter, it can interact with one of the electrons. The laws of conservation of energy and momentum apply, as in any elastic collision between two particles. This yields the change in wavelength of the scattered photon, known as the Compton effect: Ee, pe Ep’ , pp ’ Read: section 3.4 Kane Pair Production and Annihilation In 1932, C. D. Anderson observed a positively charged electron (e+) in cosmic radiation. This particle, called a positron, had been predicted to exist several years earlier by P. A. M. Dirac. A photon’s energy can be converted entirely into an electron and a positron in a process called pair production: Paul Dirac (1902 - 1984) Pair Production in Empty Space h Conservation of energy for pair production in empty space is: E E+ h E E The total energy for a particle is: So: E p c This yields a lower limit on the photon energy: Momentum conservation yields: h p c p c h pc cos( ) p c cos( ) This yields an upper limit on the photon energy: h p c p c A contradiction! And hence the conversion of energy and momentum for pair production in empty space is impossible! Pair Production in Matter In the presence of matter, the nucleus absorbs some energy and momentum. The photon energy required for pair production in the presence of matter is: h E E K .E.(nucleus) h 2me c 2 1.022 MeV Pair Annihilation A positron passing through matter will likely annihilate with an electron. The electron and positron can form an atom-like configuration first, called positronium. Pair annihilation in empty space produces two photons to conserve momentum. Annihilation near a nucleus can result in a single photon. hv hv Pair Annihilation Conservation of energy: 2me c 2 hv1 hv2 Conservation of momentum: hv1 hv2 0 c c So the two photons will have the same frequency: v1 v2 v The two photons from positronium annihilation will move in opposite directions with an energy: hv me c 2 0.511 MeV hv hv What is a photon? Photons move at the speed of light, just like an electromagnetic wave They have zero rest mass and rest energy They carry energy and momentum E=h and p=h/ They can be created and destroyed when radiation is emitted or absorbed They can have particle-like collisions with other particles such as electrons Probability to observe photons|electric field amplitude|2 Indeed, photographs taken in dimmer light look grainier. Very very dim Bright Very dim Very bright Dim Very very bright When we detect very weak light, we find that it’s made up of particles. We call them photons. CHAPTER 4 Wave Properties of Particles 4.1 4.2 4.3 4.4 4.6 4.8 4.7 X-Ray Scattering De Broglie Waves Electron Scattering Wave Motion Uncertainty Principle Particle in a Box Probability, Wave Functions, and the Copenhagen Interpretation 4.5 Waves or Particles? Louis de Broglie (1892-1987) I thus arrived at the overall concept which guided my studies: for both matter and radiations, light in particular, it is necessary to introduce the corpuscle concept and the wave concept at the same time. - Louis de Broglie, 1929 Prof. Rick Trebino, Georgia Tech, www.frog.gatech.edu X-Ray Scattering (Diffraction) In 1912, Max von Laue suggested that, since x-rays were a form of electromagnetic radiation, they should diffract. Crystals have interatomic separations similar to x-ray wavelengths and so act as three-dimensional diffraction gratings, diffracting x-rays. Bragg’s Law William Lawrence Bragg showed that x-ray diffraction acted like reflection from planes of atoms in the crystal. There are two conditions for constructive interference of the scattered x-rays: 1) The angle of incidence must equal the angle of reflection. 2) The difference in path lengths must be an integral number of wavelengths. Bragg’s Law: n = 2d sin (n = integer) The Bragg Spectrometer A Bragg spectrometer diffracts x-rays from a crystal. It measures the intensity of the diffracted beam vs. angle. When x-rays pass through a powdered crystal, the dots become a series of rings. De Broglie Waves In his thesis in 1923, Prince Louis V. de Broglie suggested that mass particles should have wave properties similar to electromagnetic radiation. The energy can be written as: h = pc If a light-wave could also act like a particle, why shouldn’t matter-particles also act like waves? h = p The wavelength of a matter wave is called the de Broglie wavelength: h/ p So do experiments confirm this idea? Louis V. de Broglie (1892-1987) Electron Scattering George P. Thomson (1892–1975), son of J. J. Thomson, saw electron diffraction from celluloid, gold, aluminum, and platinum. A randomly oriented polycrystalline sample of SnO2 produces rings. In 1925, Davisson and Germer observed electrons diffracting (much like x-rays) from nickel crystals. Beautiful Proof That Electrons are Waves: Imaging Using Them Imaging using light waves is well known. But optical microscopes’ resolution is only /2 ~ 200nm. Electron micrograph of pollen grains with ~0.1nm resolution Electrons have much smaller wavelengths, and electron microscopes can achieve resolutions of ~0.05nm. Recall that waves diffract through slits. Fraunhofer diffraction patterns One slit Two slits In 1803, Thomas Young saw the two-slit pattern for light, confirming the wave nature of light. But particles are also waves. So they should exhibit similar patterns when passing through slits, especially pairs of slits. Electron Double-Slit Experiment C. Jönsson of Tübingen, Germany, succeeded in 1961 in showing double-slit interference effects for electrons by constructing very narrow slits and using relatively large distances between the slits and the observation screen. This experiment demonstrated that precisely the same behavior occurs for both light (waves) and electrons (particles).