* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CHAPTER 3: The Experimental Basis of Quantum
Standard Model wikipedia , lookup
ATLAS experiment wikipedia , lookup
Future Circular Collider wikipedia , lookup
ALICE experiment wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Quantum vacuum thruster wikipedia , lookup
Monte Carlo methods for electron transport wikipedia , lookup
Nuclear structure wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Old quantum theory wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Elementary particle wikipedia , lookup
Atomic nucleus wikipedia , lookup
Photoelectric effect wikipedia , lookup
Photon polarization wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Electron scattering wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
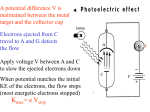
CHAPTER 3 Prelude to Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X-Ray and the Electron Determination of Electron Charge Line Spectra Quantization Blackbody Radiation Photoelectric Effect X-Ray Production Compton Effect Pair Production and Annihilation Max Karl Ernst Ludwig Planck (1858-1947) Problems due Wednesday Sept. 24th: Chapter 3: 7, 9, 14, 15, 16, 18, 19, 24, 25, 26 Photons also have momentum! Use our expression for the relativistic energy to find the momentum of a photon, which has no mass: E (mc ) p c 2 2 2 Alternatively: When radiation pressure is important: 2 2 E h h p c c h 2 p k 2 Comet tails (other forces are small) Viking spacecraft (would've missed Mars by 15,000 km) Stellar interiors (resists gravity) 3.8: Compton Effect Ep, pp Photons have energy and momentum: E hc / p h/ When a photon enters matter, it can interact with one of the electrons. The laws of conservation of energy and momentum apply, as in any elastic collision between two particles. This yields the change in wavelength of the scattered photon, known as the Compton effect: Ee, pe Ep’ , pp ’ Read: section 3.4 Kane 3.9: Pair Production and Annihilation In 1932, C. D. Anderson observed a positively charged electron (e+) in cosmic radiation. This particle, called a positron, had been predicted to exist several years earlier by P. A. M. Dirac. A photon’s energy can be converted entirely into an electron and a positron in a process called pair production: Paul Dirac (1902 - 1984) Pair Production in Empty Space h Conservation of energy for pair production in empty space is: E E+ h E E The total energy for a particle is: So: E p c This yields a lower limit on the photon energy: Momentum conservation yields: h p c p c h pc cos( ) p c cos( ) This yields an upper limit on the photon energy: h p c p c A contradiction! And hence the conversion of energy and momentum for pair production in empty space is impossible! Pair Production in Matter In the presence of matter, the nucleus absorbs some energy and momentum. The photon energy required for pair production in the presence of matter is: h E E K .E.(nucleus) h 2me c 2 1.022 MeV Pair Annihilation A positron passing through matter will likely annihilate with an electron. The electron and positron can form an atom-like configuration first, called positronium. Pair annihilation in empty space produces two photons to conserve momentum. Annihilation near a nucleus can result in a single photon. hv hv Pair Annihilation Conservation of energy: 2me c 2 hv1 hv2 Conservation of momentum: hv1 hv2 0 c c So the two photons will have the same frequency: v1 v2 v The two photons from positronium annihilation will move in opposite directions with an energy: hv me c 2 0.511 MeV hv hv CHAPTER 4 Structure of the Atom 4.1 The Atomic Models of Thomson and Rutherford 4.2 Rutherford Scattering 4.3 The Classic Atomic Model 4.4 The Bohr Model of the Hydrogen Atom 4.5 Successes & Failures of the Bohr Model 4.6 Characteristic X-Ray Spectra and Atomic Number 4.7 Atomic Excitation by Electrons Niels Bohr (1885-1962) The opposite of a correct statement is a false statement. But the opposite of a profound truth may well be another profound truth. An expert is a person who has made all the mistakes that can be made in a very narrow field. Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr Structure of the Atom Evidence in 1900 indicated that the atom was not a fundamental unit: 1) There seemed to be too many kinds of atoms, each belonging to a distinct chemical element (way more than earth, air, water, and fire!). 2) Atoms and electromagnetic phenomena were intimately related (magnetic materials; insulators vs. conductors; different emission spectra). 3) Elements combine with some elements but not with others, a characteristic that hinted at an internal atomic structure (valence). 4) The discoveries of radioactivity, x rays, and the electron (all seemed to involve atoms breaking apart in some way). Knowledge of atoms in 1900 Electrons (discovered in 1897) carried the negative charge. Electrons were very light, even compared to the atom. Protons had not yet been discovered, but clearly positive charge had to be present to achieve charge neutrality. Thomson’s Atomic Model Thomson’s “plum-pudding” model of the atom had the positive charges spread uniformly throughout a sphere the size of the atom, with electrons embedded in the uniform background. In Thomson’s view, when the atom was heated, the electrons could vibrate about their equilibrium positions, thus producing electromagnetic radiation. Unfortunately, Thomson couldn’t explain spectra with this model. Experiments of Geiger and Marsden Rutherford, Geiger, and Marsden conceived a new technique for investigating the structure of matter by scattering a particles from atoms. Experiments of Geiger and Marsden 2 Geiger showed that many a particles were scattered from thin gold-leaf targets at backward angles greater than 90°. Electrons can’t back-scatter a particles. Before After Calculate the maximum scattering angle— corresponding to the maximum momentum change. It can be shown that the maximum momentum transfer to the a particle is: Dpmax 2me va Determine max by letting Dpmax be perpendicular to the direction of motion: max Dpa 2me va pa M a va too small!