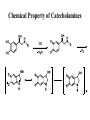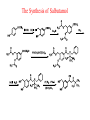* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Adrenergic Receptor Agonists
Survey
Document related concepts
Pharmacognosy wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
Drug interaction wikipedia , lookup
Epinephrine autoinjector wikipedia , lookup
NMDA receptor wikipedia , lookup
Toxicodynamics wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Norepinephrine wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Neuropharmacology wikipedia , lookup
Transcript
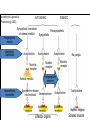
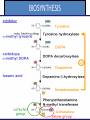
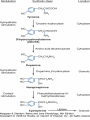
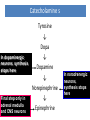
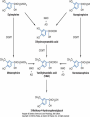
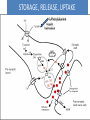
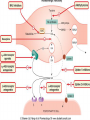
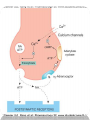
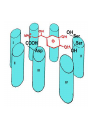
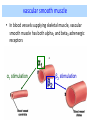
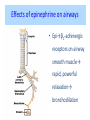
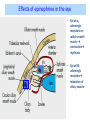
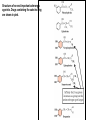
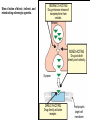
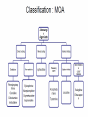
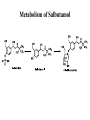
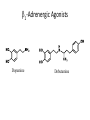
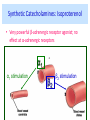
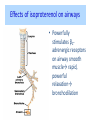
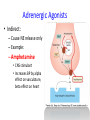
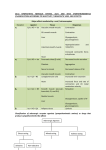
Adrenergic Nervous System Lec 1-3 By Nohad AlOmari Atrushi SYMPATHOMIMETIC – Sympathomimetic drugs mimic the effects of sympathetic nerve stimulation – Also adrenergic agonists – Adrenergic receptors Summary of the neurotransmitters released and the types of receptors found within the autonomic and somatic nervous system. (according to Lippincott´s Pharmacology, 2006) Preganglionic neuron Ganglionic transmitter AUTONOMIC Sympathetic innervation Parasympathetic of adrenal medulla Sympathetic Acetylcholine Acetylcholine Nicotinic receptor Adrenal medulla Neuroeffector transmitter SOMATIC No ganglia Nicotinic receptor Nicotinic receptor Postganglionic neurons Epinephrine release into the blood Norepinephrine Adrenergic receptor Acetylcholine Adrenergic receptor Effector organs Acetylcholine Muscarinic receptor Acetylcholine Nicotinic receptor Striated muscle Adrenergic Agonists • Chemical classification – Catecholamines – Non-catecholamines Adrenergic Agonists Differences Catecholamine – Cannot use orally – Cannot cross BBB Short-half live Examples: Norepinephrine Epinephrine Isoproteronol Dopamine Dobutamine Noncatecholamines • Can use orally • Can cross BBB Longer half life Examples: Ephedrine Phenylephrine Terbutaline ENDOGENOUS CATECHOLAMINES Naturally occurring in body: Norepinephrine -Sites: postganglionic sympathetic sites (except sweat glands , erector pillorie, hair follicles, ) • Epinephrine- Secreted by adrenal medulla • Dopamine- Major transmitter in basal ganglia, CTZ, limbic system, anterior pitutory. BIOSYNTHESIS Catecholamine s In dopaminergic neurons, synthesis stops here Final step only in adrenal medulla and CNS neurons Tyrosine ↓ Dopa ↓ Dopamine ↓ Norepinephrine ↓ Epinephrine In noradrenergic neurons, synthesis stops here STORAGE, RELEASE, UPTAKE L-Phenylalanine Hepatic hydroxylase Adrenergic Receptors • These are mainly 2 types (α) Alfa (β) Beta These are again subdivided into various types ADRENERGIC RECEPTORS ADRENERGIC DRUGS Symphathomimetics • DIRECT SYMPHATHOMIMETICS • • • • • • • Adrenaline Noradrenaline Isoprenaline Phenylephrine Methoxamine Salbutamol Xylometazoline • INDIRECT SYMPHATHOMIMETICS • Tyramine • Amphetamine • MIXED SYMPHATHOMIMETICS • Ephedrine • Dopamine • Mephenteramine THERAPEUTIC CLASSIFICATION OF ADRENERGIC DRUGS Pressor agents Noradrenaline Phenylephrine Ephedrine Methoxamine Dopamine Mephentermine Cardiac stimulants Adrenaline Isoprenaline Dobutamine Bronchodilators Adrenaline Isoprenaline Salbutamol (Albuterol) Terbutaline Salmeterol Formoterol Bambuterol Nasal decongestants Phenylephrine Xylometazoline Oxymetazoline Naphazoline Pseudoephedrine Phenyl propanolamine CNS stimulants Amphetamine Dexamphetamine Methamphetamine Anorectics Fenfluramine Dexfenfluramine Sibutramine Uterine relaxant and vasodilators Ritodrine Isoxsuprine Salbutamol Terbutaline PHARMACOLOGICAL ACTIONS Cardiac effects • Positive chronotropic effect – An action that increases heart rate • Positive dromotropic effect – An action that speeds conduction of electrical impulses (↑ conduction velocity through AV node) • Positive inotropic effect – An action that increases the force of contraction of cardiac muscle Cardiac effects of epinephrine Cardiac output is determined by heart rate and stroke volume CO = HR x SV Epi→ β1receptors at SA node→↑HR Epi→ β1receptors on ventricular myocytes→ ↑ force of contraction vascular smooth muscle α1 • In blood vessels supplying skin, mucous membranes, viscera and kidneys, vascular smooth muscle has almost exclusively alpha1-adrenergic receptors • Also biphasic response vascular smooth muscle • In blood vessels supplying skeletal muscle, vascular smooth muscle has both alpha1 and beta2 adrenergic receptors α1 α1 stimulation β2 β2 stimulation Effects of epinephrine on blood vessel caliber α1 • Blood vessels to skin, mucous membranes, viscera and kidneys • Stimulation of α1adrenergic receptors causes constriction of vascular smooth muscle Effects of epinephrine on blood vessel caliber: skeletal muscle • At low plasma concentrations of Epi, β2 effect predominates→ vasodilation • At high plasma concentrations of Epi, α1 effect predominates→ vasoconstriction α1 β2 Effects of Epi on arterial blood pressure Arterial BP = CO x PVR Epinephrine: – ↑ CO – Low doses ↓ PVR (arteriolar dilation in skeletal muscle) – High doses ↑PVR Effects of epinephrine on airways • Epi→β2-adrenergic receptors on airway smooth muscle→ rapid, powerful relaxation→ bronchodilation Effects of epinephrine in the eye • Epi at α1adrenergic receptors on radial smooth muscle → contraction→ mydriasis α1 β2 • Epi at B2adrenergic receptors→ relaxation of ciliary muscle Mnemonic for therapeutic uses of adrenaline ABCDEG A- Anaphylactic shock B- Bronchial asthma C- Cardiac arrest D- Delay absorption of local anesthetics E- Epistaxis, Elevate BP G- Glaucoma Others : Reduce nasal congestion, Induces mydriasis Therapeutic uses • Shock (moderate doses) – ↑ blood flow to kidney and mesentery – ↑ cardiac output • Refractory congestive heart failure – Moderate doses ↑ cardiac output without ↑PVR Structures of Norepinephrine and Epinephrine Norepinephrine Epinephrine (Adrenaline) •Belong to chemical class of substances known as the Catecholamines. •Polar compounds, containing both basic and acidic functional groups. •Chiral compounds. Natural enantiomer has R-configuration. •Undergoes oxidation in prolonged exposure to air. •Limited therapeutic use. Chemical Property of Catecholamines Structures of several important adrenergic agonists. Drugs containing the catechol ring are shown in pink. (according to Lippincott´s Pharmacology, 2006) Structure-activity relationships among catecholamines and related compounds Selective b-agonists OH HO Selective a-agonists N HO N HO HO a-Me-noradrenaline N HO HO OH Metaraminol N HO HO Propranolol OH HO HO N Adrenaline N Non-selective agonist Noradrenaline N Dopamine N HO N O Isoprenaline OH HO HO OH OH OH HO Selective b-antagonists Salbutamol Tyramine OH N Ephedrine Indirectly acting sympathomimetic amines N Amphetamine Norepinephrine 4-((R)- 1-hydroxy-2-aminoethyl)- 1,2-benzenediol •Potent stimulant of both α and β adrenoceptors •Limited therapeutic value •Used to maintain blood pressure in acute hypotensive states •Substrate for MAO and COMT, not effective orally •Chiral compounds. Natural enantiomer has R-configuration. Racemization occurs in heat or acid condition. •Undergoes oxidation in prolonged exposure to air. Sodium bisulfite used as antioxidant in NE preparations Epinephrine • Potent stimulant of both α and β adrenoceptors; • Drug of choice for reversal of acute hypersensitivity reactions (anaphylaxis, • Enhances the action of local anesthetics (restricts local blood flow); • Poor oral absorption. Rapidly metabolized by MAO and COMT; • Degrades on exposure to air and light; • Serious side effects include cerebral hemorrhage and cardiac arrhythmias . ALPHA1 AGONISTS Direct Acting Agents • These are agents which directly active the alpha1 adrenergic receptor. They are less potent than the endogenous agonists epinephrine or norepinephrine. However, because of structural modifications they are orally active and have longer plasma half-lives. There are 2 structural classes of alpha1 agonists the phenylethylamines which are close structural analogs of epinephrine and norepinephrine and the structurally unrelated imidazolines. The major action of these agents is to produce alpha1-receptor mediated vasoconstriction. SAR of Direct. general, a primary or secondary aliphatic amine separated by 2 carbons from a substituted benzene ring is minimally required for high agonist activity -OH Substituent: 1. By default must be present at β position to primary amine (i.e. spaced 2 carbons away). 2. being a chiral center the 1 position must be in R configuration for maximum activity (but many drugs sold as racemic i.e. R/S mixture)- exception dobutamine. Amine moiety as the size increases – selectivity for β receptor increases over α receptors. 2. α and β activity is maximum when R1= methyl (e.g. epinephrine). 3. R1= isopropyl (e.g. isoproterenol) – α activity is negligible and only β activity present. Benzene substituted 3′,4′- dihydroxy substitued benzene ring provides excellent receptor activity for both α and β sites. 2. However, there is poor oral bioavailablity due to COMT Sites of action of direct-, indirect-, and mixed-acting adrenergic agonists. INDIRECT-ACTING Drug enhances release of norepinephrine from vesicles. Neuron MIXED-ACTING Drug acts both directly and indirectly. Synapse DIRECT-ACTING (according to Lippincott´s Pharmacology, 2006) Drug directly activates receptor. Postsynaptic target cell membrane • • • • Phenylethylamines Phenylephrine Pseudoephedrine Methoxamine, • • • • • • Imidazolines Oxymetazoline Naphazoline Metaraminol Tetrahydrozoline Tetrahydrozoline α-Adrenergic Receptor Agonists α1−Selective Adrenergic Agonists: • Stimulation of vascular smooth muscle. • Maintenance of blood pressure in hypotension or shock Phenylephrine Methoxamine Metaraminol b-Adrenergic Receptor Agonists Most of the b-selective adrenergic agonists are used primarily as bronchodilators in asthma and other constrictive pulmonary conditions. Isoprenaline •Highly potent bronchodilator •Non-selective (β1 and β2) and leads to cardiac stimulation caused by its β1activity. •Metabolized primarily by COMT (Poor substrate for MAO) b2-Agonists (Phenylethanolamines) •Primarily used as bronchodilators in the treatment of asthma •Selectivity (β1 vs. β2) is poor at high doses •Administration by inhalation (aerosol, enhances β2-selectivity (pulmonary) Specific agents: Salbutamol, Salmeterol Salbutamol (Albuterol) 2-[(1,1-Dimethylethyl)amino]-1-[4-hydroxy-3- (hydroxymethyl)phenyl]ethanol •Selective β2 agonist •Orally active drugs, also available for inhalation therapy; •Not metabolized by MAO or COMT; •Bronchodilator used for the treatment of asthma, chronic bronchitis, and other breathing disorders The Synthesis of Salbutamol Metabolism of Salbutamol Salmeterol •Long lipophilic substituent •Prolonged duration of action (~ 12 hrs) •Slow onset (not suitable alone for prompt relief of bronchospasm, b1-Adrenergic Agonists Dopamine Dobutamine Dopamine •Not strictly an adrenergic drug, acts on dopamine receptors. •Stimulates cardiac b1-AR through both direct and indirect mechanisms. •Used to correct hemodynamic imbalances induced by shock, trauma, or congestive heart failure. •Rapidly metabolized by MAO and COMT. Not effective orally. Indirect Acting Agents • These agents require the presence of endogenous monoamine neurotransmitters (norepinephrine, epinephrine, dopamine, serotonin) to produce their effects. Indirect acting agonists work at the nerve terminal to promote the release and/or block the reuptake of endogenous neurotransmitters. These agents have little activity if these neurotransmitters are depleted. Cocaine and amphetamine interact with cell surface monoamine transporters for dopamine (DAT), serotonin (SERT) and norepipephrine (NET). These transporters are expressed peripherally and in specific brain loci and are the site of action of psychostimulants and antidepressant drugs. • Amphetamine: Promotes the release of monoamines from nerve endings from the terminal cytoplasm. There is only a limited amount of neurotransmitter in this pool. Amphetamine also blocks the reuptake of monoamines. Several structural analogs of amphetamine and "amphetamine like" agents are available for clinical use. These include: • Dexamphetamine (the resolved and more potent disomer of amphetamine) • Hydroxyamphetamine • Methamphetamine • Methylphenidate Sympathomimetics with Mixed Mechanism of Action Ephedrine Hydrochloride (1R,2S)-2-methylamino-1-phenylpropan-1-ol hydrochloride Actions and Uses ① not be affected by COMT, prolonged duration Decreased molecular polarity, easily enter CNS, cause stimulation. ② :a-methyl group, not be easily affected by MAO. prolonged duration, decreased molecular polarity, increased CNS toxicity. Ephedrine Hydrochloride •Natural product, isolated from various species of Ephedra. •Long history of use in traditional Chinese medicine (Ma Huang) •Four isomers of Ephedrine (-)-Ephedrine (+)-Ephedrine (+)-Pseudoephedrine (-)-Pseudoephedrine (+)-Pseudoephedrine • An alkaloid. A diastereoisomer of ephedrine, • Used as a nasal decongestant (many OTC preparations available) • To be used with caution in hypertensive individuals Enantiomer: Synthetic Catecholamines: Isoproterenol • Very powerfully stimulates β1- and β2-adrenergic receptors – No significant effect at α1-adrenergic receptors β1 SA nodal cells→↑HR β1 AV nodal cells→↑ conduction velocity β1 Ventricular muscle cells→ ↑ force of contraction Synthetic Catecholamines: Isoproterenol • Very powerful β-adrenergic receptor agonist; no effect at α-adrenergic receptors α1 α1 stimulation β2 β2 stimulation Isoproterenol ↓arterial BP = ↑CO x ↓↓PVR Decreased arterial blood pressure triggers autonomic reflex arc Effects of isoproterenol on airways • Powerfully stimulates β2adrenergic receptors on airway smooth muscle→ rapid, powerful relaxation→ bronchodilation Synthetic Catecholamines: Dobutamine • It’s a derivative of DA but not a D1 or D2 receptor agonist • Stimulates β1- and β2-adrenergic receptors, but at therapeutic doses, β1-effects predominate • Increases force of contraction more than increases heart rate ↑CO = ↑HR x ↑ ↑ SV Dobutamine: Therapeutic uses • • • • Shock MI Cardiac surgery Refractory congestive heart failure Major toxic effects of catecholamines • All are potentially arrhythmogenic – Epi and isoproterenol more arrhythmogenic than dopamine and dobutamine • Some can cause hypertension • Epinephrine, in particular, can cause CNS effects – fear, anxiety, restlessness • Dobutamine can cause vomiting and seizures in cats – must be used at very low doses Adverse effects • CNS: • Restlessness • Palpitation • Anxiety, tremors • CVS: • Increase BP….cerebral haemmorrhage • Ventricular tachycardia, fibrillation • May precipitate angina or AMI Non-catecholamine direct-acting adrenergic agonists Ephedrine Stimulates α1-, β1 and β2-adrenergic receptors and ↑ NE release from noradrenergic fibers Repeated injections produce tachyphylaxis It is resistant MAO, orally Longer acting (4-6), cross BBB – Marketed as dietary supplement promoted to aid weight loss, ↑ sports performance and ↑ energy. Ingredient in OTC nasal decongestants and bronchodilators Uses : Mild chronic Bronchial asthma, hypotension during spinal anaesthesia ,occasionally for postural hypotension – Sale prohibited by FDA in 2004 due to risks of life-threatening cardiac arrhythmias, stroke and death Non-catecholamine direct-acting adrenergic agonists: Phenylpropanolamine (PPA) • Actions much like ephedrine in the PNS • In veterinary medicine, used to treat urinary incontinence in dogs • Available in OTC products for treatment of nasal decongestion and as appetite suppressant • FDA has requested all companies discontinue marketing products containing PPA due to risk of hemorrhagic stroke β2-selective adrenergic agonists • Due to selectivity for β2 receptors at recommended doses, little-to-no direct stimulation of β1 receptors in heart • Inhalant administration maximizes local effect and minimizes systemic effects Mephenteramine • It is mixed sympathomimetic • COP, BP, heart rate are increased • Active orally with longer DOA (2-6 hrs),can crosses BBB Uses: To treat hypotension due to spinal anesthesia and surgical procedures Shock in MI and other hypotensive states Adrenergic Agonists • Indirect: – Cause NE release only – Example: – Amphetamine • CNS stimulant • Increases BP by alpha effect on vasculature, beta effect on heart Summary of blocking agents and drugs affecting neurotransmitter uptake or release Adrenergic blockers a - blockers Doxazosin Phenoxybenzamine Phentolamine Prazosin Tamsulosin Terazosin b - blockers Acebutolol Atenol Carvediol Esmolol Labetalol Metoprolol Nadolol Pindolol Propranolol Timolol Drug affecting neurotransmitter uptake or release (according to Lippincott´s Pharmacology, 2006) Cocaine Guanethidine Reserpine