* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Genetic Insights Into Comparative Morphology
Epigenetics of neurodegenerative diseases wikipedia , lookup
X-inactivation wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene desert wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
History of genetic engineering wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Minimal genome wikipedia , lookup
Genome evolution wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Ridge (biology) wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genome (book) wikipedia , lookup
Microevolution wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Genomic imprinting wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
Gene expression programming wikipedia , lookup
Gene expression profiling wikipedia , lookup
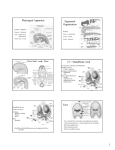
DEVELOPMENTAL DYNAMICS 209:139–155 (1997) REVIEW ARTICLE Developmental Patterning and Evolution of the Mammalian Viscerocranium: Genetic Insights Into Comparative Morphology SHIGERU KURATANI,* ISAO MATSUO, AND SHINICHI AIZAWA Department of Morphogenesis, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, Kumamoto 860, Japan ABSTRACT The vertebrate cranium is generally classified into the neurocranium and the viscerocranium. The latter is derived from the neural crest and so is the prechordal portion of the neurocranium. A view we favor considers the prechordal neurocranium as the premandibular component of the viscerocranium, and the vertebrate skull to consist of the neural crest-derived viscerocranium and the mesodermal neurocranium. Of these developmental units, only the viscerocranium appears to have completely segmented metamerical organization. The Hox code which is known to function in specification of the viscerocranium does not extend rostrally into the mandibular and premandibular segments. By genetic manipulation of rostrally expressed nonHox homeobox genes, the patterning mechanism of the head is now demonstrated to be more complicated than isomorphic registration of the Hox code to pharyngeal arches. The phenotype by haplo-insufficiency of Otx2 gene, in particular, implies the premandibular cranium shares a common specification mechanism with the mandibular arch. Our interpretation of the metamerical plan of the viscerocranium offers a new scheme of molecular codes associated with the vertebrate head evolution. Dev. Dyn. 209:139–155, 1997. r 1997 Wiley-Liss, Inc. Key words: cranium; homeobox genes; pharyngeal arch; mandibular arch; trabecula; comparative embryology; evolution INTRODUCTION Morphological and Developmental View of the Vertebrate Cranium The cranium comprises the most complicated part of the vertebrate body and has long stimulated questions as to how this structure is constructed and how it develops during ontogeny. These questions are natur 1997 WILEY-LISS, INC. rally bound to its segmental plan and its evolutionary origin. The concept of segmental vertebrate head stems from Goethe (1790), Oken (1807), and Owen (1866) who speculated that the vertebrate skull was composed of a certain number of fused vertebrae (Fig. 1A). This view is now accepted only in the occipital region that arises from somites (reviewed by Noden, 1988; Couly et al., 1993), but the basic concepts of the metamerism and metamorphosis of the cranium are still valid in the pharyngeal portion of the skull since branchial arch cartilages are also repeating units along the anteroposterior axis. Anatomically, the vertebrate cranium is divided into the brain case (neurocranium) and the pharyngeal arch skeletons (viscerocranium; Fig. 1B; see e.g., Portmann, 1976; and Torrey and Feduccia, 1979). These neuroand viscerocrania contain cartilaginous elements comprising the chondrocranium. The dermal exoskeleton covers the entire chondrocranium as dermatocranium and is also divided dorsoventrally into neuro- and viscerocranial elements. The neurocranium is regarded as the rostral continuation of the vertebral column containing the central nervous system. To this the cartilaginous sensory capsules, the nasal, optic, and otic capsules are attached. The main portion of the neurocranium is the sphenoid bone and the dermal calvarium, and the caudal portion is the occipital, which is thought to be the secondarily attached vertebrae (Fig. 1B; Fürbringer, 1897; reviewed by de Beer, 1937; Goodrich, 1930). The viscerocranium is composed of a series of repeated bar-like cartilages within the pharyngeal arches. The rostral-most element is called the mandibular arch skeleton and is altered to function as upper and lower jaws in gnathostomes. Experimental embryology has revealed that the vertebrate head contains two types of mesenchyme as the *Correspondence to: Shigeru Kuratani, Department of Morphogenesis, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, 4-24-1, Kuhonji, Kumamoto, Kumamoto 860, Japan. E-mail: [email protected] Received 26 September 1996; Accepted 25 February 1997 A eye inner ear nasal organ B neurocranium orbital region otic capsule nasal capsule notocord pharynx dermocranium gill mandibular hyoid arch arches arch viscerocranium C orbital cartilage nasal capsule quadrate cartilage otic capsule occipital cartilage vertebrae trabecula Meckel's cartilage hyoid arch branchial arches Fig. 1. Morphological plans of the cranium. A: Idealistic vertebrate as segmental organism, redrawn from Owen (1866). B: Generally accepted architecture of the vertebrate cranium, redrawn from Torrey and Feduccia (1979). The skull of gnathostomes consists of neurocranium, viscerocranium, and dermatocranium. The neurocranium contains the brain inside and the viscerocranium covers the pharynx. The latter further consists of metamerical series of cartilage bars of which the rostralmost element is parachordal cartilage notocord changed into the jaw. Sensory capsules are attached to the neurocranium. These cranial elements are cartilaginous and the whole chondrocranium is covered by dermal elements, the dermatocranium. Note the caudal portion of the neurocranium is actually the fused vertebrae, the occipital. C: Early embryonic chondrocranium of a lizard, redrawn from Torrey and Feduccia (1979). Note the position of prechordal cartilage, or the trabecula. MORPHOGENESIS OF THE MAMMALIAN SKULL source of the cranium, i.e., the neural crest-derived ectomesenchyme and mesoderm. Mesodermal mesenchyme is dorsally located as cranial paraxial mesoderm lateral to the neural tube, and ventrally, the ectomesenchyme resides in the pharyngeal arches thereby forming pharyngeal arch skeletons (reviewed by Noden, 1988; Köntges and Lumsden, 1996; Couly et al., 1996). Thus, all the viscerocranial elements (both cartilaginous and dermal) are of neural crest-origin (Le Lièvre and Le Douarin, 1975; reviewed by Noden, 1988; Couly et al., 1993). Comparative embryology can, to some degree, predict mesenchymal origins of mammalian cranial elements (see Fig. 2B). The origin of dermal bones in the calvarium still remains a matter of controversy (Le Lièvre, 1978; Noden, 1988; Couly et al., 1993). As for the origin of laryngeal cartilages in amniotes, Gegenbaur (1898; also see Starck, 1979) assumed their homologies with caudal branchial arch cartilages, which seems unlikely. It has been shown in avian embryos that these cartilages including tracheal rings are derived from the lateral mesoderm; they are more likely to represent neomorphic structures (Noden, 1983b). In the mammalian cranium, therefore, the caudalmost pharyngeal arch cartilage appears to be the caudal part of the hyoid body that belongs to the arch 3 region. On the Trabecula Cranii The mesodermal mesenchyme does not exist rostrally beyond the level of the hypophysis; the notochord, which is essential for chondrogenesis of mesodermal tissue, also does not exist in this area (Pourquie et al., 1993). Data obtained in avian experiments confirmed that the prechordal neurocranium originates from the neural crest (Le Lièvre and Le Douarin, 1975; reviewed by Noden, 1988; Couly et al., 1993). In mammalian skulls, nasal septum, lachrymal, presphenoid, basisphenoid (in part?), and anterior portions of orbitosphenoid and frontal bones are thought to belong to the prechordal region; a part of the frontal bone may also be derived from the neural crest. The junction between the prechordal and chordal cranium lies approximately at the level of the cephalic flexure of the brain and dorsum sellae of the mammalian skull (Fig. 2B). Simultaneously, this junction corresponds to the site of orbital cartilage development, the anterior-most cartilage element that develops underneath the neural tube (Kuratani, 1989). In many vertebrates, the cartilaginous prechordal neurocranium develops from an element called the trabecular cartilage that originally arises as a pair of bar-like cartilages (Fig. 1C; Rathke, 1839; reviewed by de Beer, 1931, 1937). The neural crest-origin of the trabecula in various vertebrates (Stone, 1926; Raven, 1931; Andres, 1949; Wagner, 1949, 1959; Noden, 1978; Hall and Hörstadius, 1988; Couly et al., 1993), its topographical location, morphology, and the metamerical organization of cranial nerves suggest that this cartilage may represent a premandibular component of the viscerocranium that 141 belongs to the same segment as the ophthalmic nerve, as was first stated by Huxley (1874; reviewed by Goodrich, 1930; de Beer, 1931, 1937; also see Stadmüller, 1936). Although several arguments exist, we favor this view based on the recent genetic data stated below. Considering an ammocoete larva of the lamprey as a hypothetical intermediate state, de Beer (1931) viewed the mucocartilage in the upper lip as the trabecula homologue and tried to explain how the premandibularly located branchial cartilage could have moved into the position of the prechordal neurocranium. He assumed a great change in the topographical relationship between the neural tube and the cartilaginous component to explain how the trabecula had come into contact with the nasal epithelium, hypophysis, and the forebrain. The common anatomical plan of the rostral-most neural tube is shared by a closely related group of vertebrates, the amphioxus (Lacalli et al., 1994); it is hardly conceivable that such a substantial change took place in the gnathostome evolution. Instead, Holmgren and Stensiö (1936) and others identified the ammocoete trabecula as the cartilage that develops as the rostral continuation of the parachordal, not the mucocartilage juxtaposed to the mandibula. The mucocartilage that de Beer (1931) called the trabecula does not seem to arise from the neural crest either (reviewed by Janvier, 1993). The ectomesenchyme forming the trabecula may have been originally located in close association with the rostral-most part of the gut (Allis, 1938), similar to what is the case for the other pharyngeal arch skeletal elements. In most of the early vertebrate embryos, the rostral-most portion of the gut is seen as the preoral gut located dorsoanterior to the mouth. The vertebrate mouth is formed in the ventral aspect of the pharynx and caudal to the trabecular mesenchyme (Fig. 3A). The position of the preoral gut is very close to the hypophysis, nasal epithelium, forebrain, and the ophthalmic nerve. Kastschenko (1887) reported vestigial pharyngeal pouches on the side wall of Seessel’s pouch. A reasonable hypothesis, then, is to assume that the trabecular cartilage may have grown out and slid rostrally beneath the forebrain, not swung rostrally as de Beer proposed, and became the prechordal neurocranium (Fig. 3B). The incorporation of the trabecula into the formation of the neurocranium is probably associated with the mouth formation in ancestral vertebrates, earlier than the formation of the biting jaw. The absence of dorsoventral articulation of the trabecula cranii in gnathostomes would mean that it was attached to the brain before articulation arose (also see Allis, 1938). The Hox Code in the Patterning of the Viscerocranium In 1828 Karl Ernst von Baer proclaimed the pharyngular stage as the bottleneck in vertebrate development; all the vertebrates arrive at this stage by differ- 142 KURATANI ET AL. Fig. 2. Hypothetical genetic codes of the viscerocranial patterning and embryonic composition of the mammalian skull. A: The Hox code is present in the second arch and more caudal levels. This code is parallel to that in the neuraxis, which is indicated by bars with the same colors as the mesenchymal code. The posterior portion of the first arch skeleton may be patterned by the default state of the Hox code, whereas the anterior lower portion (dentary, D) by Otx2. Crest cells from the caudal midbrain which express Otx2 fill the mandibular arch (Osumi-Yamashita et al., 1994). A3–6, pharyngeal arches; H, hyoid arch; M, the first arch; ot, otocyst; PM, premandibular segment. B: Origins of viscerocranial elements in mamma- lian skull. Colors indicate the origin of ectomesenchyme shown in (A). White represents elements whose origins are uncertain. Gray elements develop from mesodermal mesenchyme in avian embryos (Couly et al., 1993). Otic capsule (ot) is derived from both mesoderm and neural crest (Noden, 1988). as, Alisphenoid; bo, basioccipital; bs, basisphenoid; eo, exoccipital; f, frontal; i, jugal; in, incus; ip, interparietal; j, jugal; lc, lachrymal; m, malleus; mn, mandibula; mx, maxilla; nc, nasal capsule; ns, nasal; par, parietal; pl, palatine; pm, premaxilla; ps, presphenoid; pt, pterygoid; so, supraoccipital; sq, squamosal; st, stapes; ty, tympanic; vom, vomer. Fig. 3. Morphological plan of the vertebrate cranium—a hypothesis. A: Generally, the skull is assumed to be composed of dorsal neurocranium and ventral viscerocranium. The neurocranium in this scheme is subdivided into mesodermal chordal cranium and neural crest-derived prechordal cranium. B: In the present review we propose that the premandibular visceral arch element (5 the trabecula) was present in association with the preoral gut in the ancestral vertebrate. This cartilage element would have developed close to the olfactory epithelium and the hypophysis and located dorsoanterior to the mouth that opened on the ventral aspect of the pharynx. C: Developmental plan of the gnathostome cranium. The thick dotted line indicates the interface between the neuro- and viscerocrania in the concept of the present scheme, which simultaneously represents the interface between the mesodermal and crest-derived portions of the skull. Visceral arch skeletons except the premandibular one are divided into dorsal and ventral halves. The mandibular arch skeleton acquired masticatory capability. The trabecula has been changed into a secondary neurocranium. Consistent with the ancestral state, the trabecula maintains close association with the nasal epithelium. 144 KURATANI ET AL. ent means and develop different features, but there is something in this particular stage that appears invariant morphologically. It is during this period that the collinear nested pattern of Hox gene expressions, the Hox code is established along the axis (reviewed by McGinnis and Krumlauf, 1992; and by Krumlauf, 1993; Prince and Lumsden, 1994; Mark et al., 1995); those located on the more 38 side within the gene cluster are expressed from the more anterior levels of the body axis and extend caudally (Fig. 2A). In the trunk, the Hox genes are expressed in provertebrae, and shifts or modification of the Hox code result in the re-specification of vertebrae (Balling et al., 1989; Le Mouellic et al., 1992; Jegalian and De Robertis, 1992; Pollock et al., 1992; McLain et al., 1992; Lufkin et al., 1992; Charité et al., 1995; Suemori et al., 1995). Consistent results have also been obtained by the retinoic acid-induced respecification of vertebrae, which is roughly associated with the shift of the Hox code in the provertebrae (Kessel and Gruss, 1991; Kessel, 1992). It has been shown recently that multiple disruptions of paralogous Hox genes can result in more profound and wideranging transformations of vertebrae (Horan et al., 1995) or deletion of elements, probably reflecting the involvement of paralogues in different phases of cell proliferation (Condie and Capecchi, 1994). Each pharyngeal arch skeletal element also achieves its specific shape through development under the Hox code. This segmental metamorphosis has been suggested to be determined in the premigratory neural crest that extends from the mid- to hindbrain neurectoderm (Wagner, 1959). In the hindbrain, the anterior border of each Hox gene expression domain coincides with the anterior sulci of odd-numbered rhombomeres (r3, r5, and r7), the segmented bulges of the hindbrain (Hunt et al., 1991; Fig. 2A). The Hox code in the caudal head region involves the first five (Hox-1 to 5) paralogues and is also present in the pharyngeal ectomesenchyme and epithelia in a pattern parallel to that in the hindbrain (Hunt et al., 1991; Prince and Lumsden, 1994). Importantly, there is no Hox gene expressed in the arch 1 segment (Fig. 2). Such Hox gene expression patterns may be conserved in diverse vertebrate classes (e.g., Frasch et al., 1995; Morrison et al., 1995), with each cognate being linked with specific segmental identity (Burke et al., 1995; van der Hoeven et al., 1996). Crest cell influx into pharyngeal arches is the principal basis of this coincidence in segmental registration between rhombomeres and pharyngeal arch mesenchyme (Köntges and Lumsden, 1996; Couly et al., 1996). The origin and migration patterns of hindbrain crest cells are not as strictly segmented on the neuraxis as had initially been thought (Sechrist et al., 1993; Niederländer and Lumsden, 1996; also see Lumsden et al., 1991; and Graham et al., 1993, 1994; Osumi-Yamashita et al., 1996). Especially, the postotic arch mesenchyme has extensively overlapping origin on the posterior hindbrain, though the pharyngeal Hox code exists in this region (Kuratani and Wall, 1992; Shigetani et al., 1995). The arch-specific Hox gene expressions, there- fore, cannot be ascribed only to the rhombomeric origin of the crest cells; these cells can autonomously continue to express the same Hox genes with the original neurepithelium as seen in r4-derived cells destined for arch 2 (Kuratani and Eichele, 1993), but the expression can also be regulated by an environment that the crest cells encounter (Saldivar et al., 1996; Hunt et al., 1995; also see Couly et al., 1996). Nevertheless, being expressed in a nested pattern Hox genes are believed to participate in the patterning of branchiomeric structures such as skeletons, and in other pharyngeal derivatives as well (Manley and Capecchi, 1995). In the viscerocranium Hoxa-2 disruption is the only example that causes homeotic transformation of the arch skeletons (see below) and no other single Hox gene disruptions have resulted in the transformation (Chisaka and Capecchi, 1991; Chisaka et al., 1992; Lufkin et al., 1991; Ramirez-Solis et al., 1993; reviewed by Manak and Scott, 1994 and by Mark et al., 1995). In contrast to the trunk region, paralogue Hox genes have the anterior expression limits at the same rhombomeric levels (reviewed by Krumlauf, 1993): evolutionary multiplication of Hox clusters and resultant redundancy in function among paralogues (e.g., Holland et al., 1994; Manak and Scott, 1994) may have limited the apparent homeotic function to Hoxa-2 by disruption of a single gene in the viscerocranium; in the second arch Hoxa-2 is major (Rijli et al., 1993; Gendron-Maguire et al., 1993; Mark et al., 1995). Concerning the apparently limited homeotic function of cephalic Hox genes, to be kept in mind is the fact that transformation of rhombomeres has also been reported only between r2/3 and r4/5 on administration of retinoic acid (Marshall et al., 1992) and in Hoxa-1 gain of function (Zhang et al., 1994). Likewise, only the ectopic cartilage with the mandibular arch identity can be generated within the second arch by the neural crest transplantation (Noden, 1978, 1983a). The caudal head, therefore, seems to possess only a limited number of rhombomeres and branchiomeres that could homeotically transform. Of note is that there are only three segments of pharyngeal arch skeletons in the mammalian cranium (excluding the trabecula), of which the third is only vestigial as a part of the hyoid complex. More caudally, pharyngeal arches are associated with somites dorsally, but Hox genes are more or less involved in somitomeric specifications (e.g., Lufkin et al., 1991; Ramirez-Solis et al., 1993), with phenotypes comparable to those in the trunk. Though the Hox code may play essential roles in the patterning of branchiomeric structures in principle, many ambiguities remain. Evolution of the Mammalian Mandibular Arch Skeleton The mammalian cranium possesses several unique features that are not seen in other vertebrates. In particular, the first arch skeleton has gone through an extensive modification during evolution. Mammals arose as a subgroup of fossil synapsid amniotes. Nevertheless, comparative morphology with extant reptiles or MORPHOGENESIS OF THE MAMMALIAN SKULL 145 Fig. 4. Comparative morphology and evolution of the middle ear skeletons. A–C: Comparative morphology. Both the mandibular and hyoid arches possess simple skeletons that are divided into dorsal and ventral elements in shark (A). In the mandibular arch, the dorsal cartilage is called palatoquadrate (pq), and the ventral the cartilage of Meckel (Mk). The caudal ends of these cartilages articulate with each other as quadrate (q) and articular (a), respectively. The dorsal element of the hyoid arch skeleton, the hyomandibula (hm), functions as suspensorium. In the modern reptile, this cartilage element is used as sound-transmitting apparatus in the middle ear, as the columella auris (col; B). The dorsal process or the epipterygoid (ept) of the palatoquadrate originates from the suprapharyngeal element of the first arch. The same cartilage develops into the ala temporalis (at) in the mammal (C) and forms a secondary cranial base as a part of the sphenoid bone (see Fig. 5). The quadrate and articular cartilages are separated from the main portion of the first arch cartilage and translocated into the middle ear as incus and malleus, respectively. Mammalian homologue of the columella auris is called stapes (st). The ethmoid (et) and goniale (gn) are homologous with the angular (an) and prearticular (pa) in the reptilian skull (B). Note the reptilian tympanic membrane develops in the upper jaw region while that of the mammal belongs to the lower jaw. ch, Ceratohyal; lh, laterohyal; Rc, Reichert cartilage; tm, tympanic membrane. Modified from Goodrich (1930). D,E: Evolution of the middle ear from cynodont (D) to mammalian (E) conditions. Cynodont lower jaw (D) possesses a dermal element, the angular, whose ventrocaudal portion is expanded as the reflected lamina (rl); this portion is changed into the tympanic (et) of mammals (E). The articular of this animal attaches to the angular and develops the retroarticular process (rp) which alters itself into manubrium mallei (mm). d, Dentary; q, quadrate; qj, quadratejugal. After Allin (1975). sauropsids has provided the following intriguing insights into the evolution of the mandibular arch skeleton. In the viscerocranium, one of the anterior elements (the second including the trabecula) acquired the masticatory ability in the earliest gnathostomes (Fig. 3). The mandibular arch skeleton is divided into two halves, the dorsal part or the palatoquadrate and the ventral part or the cartilage of Meckel. Conspicuous is the establishment of the middle ear in this skeleton of mammals. Mammals possess three ear ossicles— malleus, incus, and stapes, while amphibians and sauropsids have only one—the columella auris (Fig. 4B, C). Of those, stapes is equivalent to the columella auris of sauropsids and to hyomandibular cartilage of fish, the dorsal element of the hyoid arch skeleton. As for the mammal-specific ossicles, malleus, and incus, their direct equivalencies apparently do not exist in other vertebrates. Reichert (1837) first demonstrated by comparative observations of various embryos that these two cartilages arose as mesenchymal condensations articulating with each other as caudal portions of both palatoquadrate and Meckel’s cartilages. Thus, malleus was homologized with articular, and the incus with the quadrate of sauropsids. This finding was further strengthened by Rabl (1887), Gaupp (1912), and by Fig. 5. Morphology and evolution of the mammalian cranial wall. Left: Comparative anatomy of the cavum epiptericum. Reptiles possess the original cartilaginous cranial wall, and a part of the mandibular arch skeleton, the epipterygoid, is located lateral to the cranial wall. The epipterygoid covers the extracranial space, the ‘‘cavum epiptericum’’ (Gaupp, 1902), containing the trigeminal ganglion. In mammals, the cartilaginous cranial wall diminishes and the secondary cranial wall is established with visceral elements (ala temporalis equivalent to the reptilian epipterygoid, and a newly formed dermal element, the alisphenoid) ventrolateral to the primary one. Note the identical topographical relationships of elements between the two animals. The mammalian cavum epiptericum containing the trigeminal ganglion thus combined lateral to the original cranial cavity. Primary and secondary cranial cavities are delineated by a meningeal membrane. Modified from Goodrich (1930). Right: Comparison of the cranial side walls in the early cynodont fossil (above) and the modern mammalian skull (below). Note the expansion of the alisphenoid (as) and incorporation of the trigeminal nerve foramina (V1 for the ophthalmic, V2 for the maxillary, V3 for the mandibular nerve). as, Alisphenoid; occ, occipital; p, parietal; pal, palatine; po, postorbital; pr, perioticum; sq, squamosal. 146 KURATANI ET AL. MORPHOGENESIS OF THE MAMMALIAN SKULL 147 Fig. 6. Cranial defects caused by the Otx2 haplo-insufficiency. Cranial defects by the haplo-insufficiency are variable (Matsuo et al., 1995). A–E: Dorsal cranial views of the wild-type (A) and Otx2 haploid deficient mice (B, C). F–J: Ventral aspects of the same skulls represented in A–E. A and F show normal configuration of the newborn murine skull. B and G show the lightest anomaly in the mutant skull. No major changes are seen except for an ectopic transverse bar of bone (arrowheads) developing on the basisphenoid (bs) and foramina in the skull base (asterisk). The arrow indicates an ectopic junction between the incus (in) and alisphenoid (as). The mandible (dentary) of this specimen appears normal. In the skull shown in C and H the transverse bar is more extensive than in B, possessing a central cartilaginous nodule (asterisk). The cranial base is split into two halves at the midline (H). Note that middle ear ossicles derived from arch 1 (in, m) and the tympanic (ty) have shifted towards the midline in association with the arrested development of the dentary. More heavily deformed skulls are shown and in these embryos the dentary is missing. The eye is also missing on the right side of one embryo (D, I) and on both sides of the other (E, J). The middle ear components derived from the mandibular arch have shifted farther medially. Arrows in A indicate the alisphenoid-incus junction as seen in anamniotes and sauropsids (Fig. 4B, C). In embryo E, the mandibular arch elements have shifted extremely medially and the tympanic has almost fused to become a transverse bar. Note that in the same skull, the stapes (st), the hyoid arch derivative, has developed in the normal place. The nasal capsule was lacking in this embryo, leaving the nasal septum (ns). acc, Alicochlear commissure; bs, basisphenoid; cc, cochlear capsule; f, frontal; fo, optic foramen; g, goniale; j, jugal; m, malleus; Mc, Meckel’s cartilage; mx, maxilla; ns, nasal septum; os, orbitosphenoid; pa, processus alaris; pl, palatine; pm, premaxilla; ps, presphenoid; pt, pterygoid; Rc, Reichert cartilage; sq, squamosal. Goodrich (1930) with various evidence from comparative zoology. In addition to the middle ear ossicles, to be noted about the mammalian mandibular arch skeleton is the metamorphosis of the sphenoid (Fig. 5). The original cartilaginous neurocranium is diminished and replaced by dermal bones in mammals. Gaupp (1902) noted that the large wing of the mammalian sphenoid or its cartilaginous precursor, ala temporalis, is equivalent to a part of the palatoquadrate (5 reptilian epipterygoid), and is secondarily attached to the central stem to function as a cranial wall below the trigeminal ganglion (Fig. 5). According to Presley and Steel (1976), the alisphenoid is a dermal bone secondarily attached to the ala temporalis, representing a neomorphic structure. The 148 KURATANI ET AL. Figure 6. mammalian skull possesses the secondary cranial wall lateral to the original one, and has expanded the cranial cavity by incorporating the extracranial space, the cavum epiptericum. Evolutionarily, the incorporation of the cavum can be traced through the synapsid fossils (Fig. 5; reviewed by Kemp, 1982; Moore, 1981; Kermack and Kermack, 1984). In addition, a series of dermal elements belonging to the maxillar segments of mammals have been expanded to form medially the secondary palate, establishing the separation of air passage from the oral cavity. Reichert’s view had a number of opponents until recently (reviewed by Strickland et al., 1962; Hanson et al., 1962; Jarvik, 1980; also see Williams and Warwick, 1990). The controversy was primarily centered on the ambiguity of the boundary between ectomesenchymal domains since the first pharyngeal slit of mammals has been extensively modified. As noted above, the Hox gene expression pattern at early stages is delineated in (Continued.) terms of single arches, and disruption of a Hox gene is expected principally to affect only those skeletal elements that are derived from a single ectomesenchymal population. Indeed, by disruption of the Hoxa-2 gene, the second arch of the mutant mouse skeleton took the shape of the mandibular arch; a homeotic transformation (Rijli et al., 1993; Gendron-Maguire et al., 1993). Malleus and incus were duplicated, being consistent with the hypothesis that these are mandibular arch components, and the duplication also occurred in parts of the sphenoid proving them to be palatoquadrate derivatives (Fig. 4C). Although the final proof has to be made by long-term neural crest labeling which is technically not available at the moment, Reichert’s homology of ear ossicles is consistent with the result of Hoxa-2 disruption as is Gaupp’s in the evaluation of the alisphenoid. The Hoxa-2 disruption approximates the genetic code of the second arch skeleton to that of the first or the ‘‘default state’’ (Mark et al., 1995), and the phenotype is MORPHOGENESIS OF THE MAMMALIAN SKULL consistent with the scheme of Hox code-mediated metamorphosis of the viscerocranium. However, the anterior portion of the mandibular arch, the dentary, was never duplicated in this mutant. This suggests the possibility that morphogenetic identity of the mandibular arch is not determined only by Hox gene expression. Otx2 Gene in Mandibular Arch Patterning Several homeobox genes have been reported to be expressed in region-specific manners in the rostral head. Mouse cognates of Drosophila head gap genes ems and otd (Cohen and Jürgens, 1990; Finkelstein and Perrimon, 1990) have been isolated as Emx1, 2, and Otx1, 2, respectively (Simeone et al., 1992). Like Hox genes, these genes are expressed in a nested pattern on the neuraxis rostral to the mid-hindbrain boundary. Remarkably, the Otx2 expression domain is posteriorly limited by the mid-hindbrain junction. The gene is also expressed transiently within the mandibular arch ectomesenchyme as well as in the frontonasal region (Ang et al., 1994). Disruption of the Otx2 gene was performed by several groups independently (Acampora et al., 1995; Matsuo et al., 1995; Ang et al., 1996). The homozygous mutation resulted in embryonic lethality around 9.0 dpc with the truncation of the neuraxis anteriorly at the level of rhombomere 2; this is probably due to Otx2 expression in cells destined for the prechordal mesoderm as suggested by the same defect in Lim-1 deficiency (Acampora et al., 1995; Shawlot and Behringer, 1995; Matsuo et al., 1995; Ang et al., 1996). Heterozygous mice with disrupted Otx2 alleles showed an interesting craniofacial haploinsufficiency phenotype (Matsuo et al., 1995). The phenotypes are reminiscent of otocephaly in man and rodents with a graded series of severity. Most striking was the loss of the dentary, and this was associated with disturbed development of mesencephalic trigeminal neurons; these neurons evolved to control the force of mastication, and agnatha which does not have a jaw also lacks the neurons (Sarnat and Netsky, 1981). The Otx2 defects did not extend more posteriorly into the middle ear components. The sphenoid was always present though sometimes deformed possibly due to defects of the premandibular segment (see below). Notable is a connection between the incus and alisphenoid that sometimes developed in Otx2 haploid mutants; the connection is reminiscent of the solid palatoquadrate in ancestral forms (Fig. 6). Phenotypes in the mandibular arch skeleton found in Otx2 haploid- and Hoxa-2 disruptions are complementary to each other: in the Hoxa-2 mutant, only the posterior component of the mandibular arch skeleton was duplicated, while in the Otx2 heterozygous mutant, only the anterior component, the dentary, tended to be lost. The ectomesenchyme in this arch originates both from rhombencephalic and mesencephalic regions (Osumi-Yamashita et al., 1994; also see Le Lièvre, 1974; Köntges and Lumsden, 1996). In addition, Dil labeling studies have suggested that crest cell populations origi- 149 nated from different levels of neuraxis do not intermingle evenly but are, rather spatially dissociated from each other within the first arch (Osumi-Yamashita et al., 1994, 1996; Imai et al., 1996). The mandibular arch mesenchyme may thus be subdivided into at least two domains. The posterior components involving the malleus, incus, tympanic, sphenoid (in part), and pterygoid may be patterned after a default state, whereas the dentary is patterned by Otx2. Homeobox Genes Involved in the Formation of Mandibular Arch Skeletons Analyses of mutant mice by gene targeting have now clarified the roles of many genes related to cranial development (Kurihara et al., 1994; Lohnes et al., 1994; Mendelsohn et al., 1994; Matzuk et al., 1995; Kaufman et al., 1995; Dietrich and Gruss, 1995; Luo et al., 1995; Schorle et al., 1996; Zhang et al., 1996; Mansouri et al., 1996), although the redundant function among related genes inherently obstructs this approach. Phenotypes were usually more limited than was expected from their expression patterns. Of those, mutant phenotypes of several homeobox genes are worth considering in the context of this review. gsc, originally identified as the gene actively expressed in the Xenopus organizer (Cho et al., 1991), is expressed in the anteriormost mesoderm during gastrulation and in the posterior half of the mandibular arch (Gaunt et al., 1993). However, gscdeficient mice showed only atrophies of the tympanic bone and ala temporalis (Yamada et al., 1995; RiveraPérez et al., 1995); both are mandibular arch-derivatives uniquely specified in mammals as noted above. MHox is a paired-like homeobox gene and expressed in undifferentiated ectomesenchyme of all the pharyngeal arches (Cserjesi et al., 1992; Kuratani et al., 1994), but the disruption of the gene in mouse leads to malformation of mandibular arch components alone (Martin et al., 1995); an ectopic process developed on Meckel’s cartilage, and ala temporalis, the jugal, tympanic, and squamosal diminished. Msx1, an msh-type homeobox gene, is strongly expressed in the distal portion of the mandibular arch (MacKenzie et al., 1991) and its disruption leads to the partial loss of the malleus and arrested development of teeth (Satokata and Maas, 1994). Dlx-2, a Distalless-related homeobox gene is expressed in the mandibular and hyoid arches (Porteus et al., 1991; Price et al., 1991; Robinson et al., 1991; Robinson and Mahon, 1994), and its disruption causes the rearrangement of dermal elements (Qiu et al., 1995). Some of the ectopically developed dermal bones in this mutant are reminiscent of the dermocranial pattern of synapsids (reviewed by Kermack and Kermack, 1984). The secondary palate is another mammalian specific feature derived from the mandibular arch as stated. Its development was disturbed and a cleft palate was produced by disruptions of Hoxa-2, Dlx-2, and Msx1. Defects in the alisphenoid by Dlx-2, gsc, and MHox mutations is consistent with the assumption that this 150 KURATANI ET AL. Fig. 7. Cranial defects due to the Otx2 haplo-insufficiency (continued). One of the most severely affected skulls is showed on the left. In this specimen the prechordal cranial base failed to fuse rostrally from the level of basisphenoid (bs), and yielded a large fossa (**). This separate portion located at the prechordal level. The pair of rod-like cartilages (*) and the fossa are reminiscent of the trabecula (tr) and hypophysial fenestra (hy), respectively, in the chondrocranium of Ambystoma at 12 mm stage (Winslow, 1898). Note the extension of the notochord (nt) in this animal. For abbreviations, see Figure 6. bone element primarily belongs to the mandibular ectomesenchyme. As noted earlier, comparative embryology suggested that in mammals the original connection between the ala temporalis and incus was lost leaving only a thread of condensed connective tissue (Presley and Steel, 1976). This connection partly chondrifies in Otx2 and Dlx-2 mutants, though differently (Fig. 6; Qiu et al., 1995). The Otx2 and Hoxa-2 genes are expressed in an axial level-specific manner in multiple cell lineages. Expressions of Dlx-2, MHox, Msx1, and gsc are more likely to be associated with mesenchymal tissue in the pharynx at later onset, and may developmentally locate downstream of axial level-specification. Their disruptions caused very limited deformities which correspond more or less to mammalian specific features of skeletons. The presence of closely related genes that are expressed in overlapping domains (s8, Dlx paralogues, Msx2) should be kept in mind. At the same time, evocation of regulatory mechanisms that recruited these genes for mammal-specific patterning and evolution of the skull might be suggested. Patterning of the Premandibular Segment In addition to the mandible, major defects by Otx2 haplo-insufficiency were found in premandibular skeletons, and were often associated with loss of the nasal Fig. 7. Fig. 8. Metamerical composition of the vertebrate viscerocranium and the genetic codes (hypothesis). Above: In the hypothetical ancestor of vertebrates the viscerocranium consisted of a series of atypical pharyngeal arch skeletons with no articulations. The mouth opened, in this animal, on the ventral aspect of the pharynx, caudal to the rostralmost segmental element. In this ancestor, the collinear expression of Hox genes and anteriorly restricted expression of head homeobox genes, Otx and Emx genes (Simeone et al., 1992; Boncinelli et al., 1993; Matsuo et al., 1995; Yoshida et al., 1997; Suda et al., 1996), may have existed. Importantly, the mandibular cartilage-equivalent (the second cartilage element from the front) is contributed by the rostral-most ectomesenchyme as well as by the second. Below: In vertebrates the compartmentrestricted Hox code and the anterior homeobox genes have enabled segment-specific homeosis between mandibular and hyoid arches. Hox code exists in r3 and caudally (r2 is secondarily involved as shown by thinner broken line; Prince and Lumsden, 1994), while the Otx2 expression domain has its caudal boundary at the mid-hindbrain boundary. Note that arches 4 to 6 do not develop pharyngeal arch skeletons in mammals (shown by open lines). The thick dotted line roughly indicates the boundary between regions in which Hox code is involved in the viscerocranial (branchiomeric) and the somitomeric specifications. The mandibular arch skeleton develops from two populations of ectomesenchyme carrying distinct genetic codes. Note that blank ectomesenchyme of the mandibular arch represents the ‘‘default’’ state; its developmental identity is mimicked by the second arch in Hoxa-2 disruption (Rijli et al., 1993; Gendron-Maguire et al., 1993) and is not affected by Otx2 haploinsufficiency (Matsuo et al., 1995). MORPHOGENESIS OF THE MAMMALIAN SKULL Fig. 8. 151 152 KURATANI ET AL. capsule (Fig. 6). In the cranial base the presphenoid was often deformed, the region was suggested to be of neural crest-origin (Couly et al., 1993) and to belong to the premandibular segment as stated above. The posterior part of the basisphenoid, on the other hand, was not affected. This part of the skull base is most likely to be of mesodermal origin as suggested by avian embryology and comparative embryology (Fig. 2B; Kuratani, 1989). Sometimes Otx2 heterozygous embryos possessed a pair of separate cartilage rods in place of the nasal septum (Fig. 7). The overall configuration resembled the platybasic skull base; the morphology of bar-shaped cartilage is widespread in anamniotes and is also shared by sauropsid chondrocrania at earliest stages but not in mammals (see de Beer, 1937). In addition, the defects were associated with specific loss of the ophthalmic branch of the trigeminal nerve that belongs to the same segment as the premandibular skeleton (Matsuo et al., 1995). The nasal capsule largely consists of the lateral nasal wall including various processes (turbinals), and the medial nasal septum, the direct derivative of the trabecula (Bellairs, 1958). The former appears to derive from the mesenchyme within the lateral nasal prominence and the latter from the medial nasal prominence (see Osumi-Yamashita et al., 1997). The lack of the nasal wall in Otx2 heterozygotes was always associated with the loss of nasal epithelium while the nasal septum was always present, though slightly deformed (Fig. 6; Matsuo et al., 1995). Strikingly similar defects have been reported in Pax 6 mutants in mouse (Kaufman et al., 1995) and in the rat (Osumi-Yamashita et al., 1997). In this mutant, a ‘‘unicorn’’-shaped medial cartilage bar representing the nasal septum developed, but no trace of lateral walls was found with the lack of nasal epithelium. Thus, it seems likely that the patterning mechanisms are different between the nasal septum and the nasal wall. Both Pax 6 and heterozygote Otx2 mutants have eye defects, but lack of the eyeball does not affect the development of the nasal cartilage elements (Bellairs, 1958). Coextensive loss of nasal epithelium and the lateral wall suggests the epithelialdependent development of this cartilage as stated by Thorogood (1988). Premandibular skeletal elements such as the lachrymal and anterior part of the frontal were not substantially deformed in Otx2 heterozygotes, though primary defects cannot definitively be discriminated from secondary defects by morphological criteria. Nevertheless, absence of any defects in the cranial base caudal to the hypophysial level along with the specific loss of the ophthalmic branch of the trigeminal nerves strongly suggests that Otx2 functions in a branchial segment specific manner which is in accordance with the segmental scheme of the viscerocranium, and is responsible for the formation of the premandibular segment (Matsuo et al., 1995). Chordate Cranial Evolution From Morphology and Molecules Master patterning genes such as Otx2 and Hox genes are expressed along the axis in a conserved nested pattern, which may be universal throughout the animal kingdom (reviewed by Slack et al., 1993; and by Holland and Garcia-Fernàndez, 1996). These genes may act only to demarcate relative positions, and under this demarcation specification of any particular structure is diverged in each group of animals. The vertebrate body plan is not simple, nor is the genetic code associated with this plan. It is also highly possible that the assignment of each unit may only be based upon topographical relationships with no profound plan. We believe that the prechordal neurocranium represents a viscerocranial element as stated above (Figs. 3, 8). The viscerocranium is metamerically organized in register with cranial nerves and pharyngeal arches. Prechordal neurocranium is the most anterior, premandibular component that belongs to the same segment as the ophthalmic nerve. It was incorporated into the neurocranium in association with the mouth formation in ancestral vertebrates before the formation of the masticatory jaw and utilized and modified to support the brain through vertebrate evolution (Figs. 3, 8). A number of events involved in the metamerical patterning of the viscerocranium are under the control of genetic codes, with the Otx2 gene expressed anteriorly and the Hox genes posteriorly (Fig. 8). A default state exists in the posterior part of the first arch being destined for middle ear structures, whose evolutionary and embryological constraints imposed on the vertebrate body plan are of great interest. The anterior part of the mandibular arch, on the other hand, is under Otx2 control and destined to develop into the mandible. Otx2 gene may have recruited a number of developmental cascades in the course of diversification of this cartilage element. At the same time, this gene may have been utilized in the masticatory system with mesencephalic trigeminal neurons and midbrain crest cells in transition from the agnatha to gnathostomata (Matsuo et al., 1995). In gnathostome history, modification of the mandibular arch skeleton was remarkable as shown by the specification of the middle ear ossicles and of the secondary cranial base in mammals. The default state for Hox or Otx genes might have some relevance to the apparently unique nature of this arch. Several non-Hox homeobox genes such as gsc, Msx, and Dlx are expressed in a wide range of pharyngeal ectomesenchyme, but their unique functions in the mandibular arch are suggested by disruptions of these genes in mice. It is noteworthy that Hoxa-2 expression has been suggested to restrict the function of gsc to the first arch (Mallo and Gridley, 1996). The default state might thus be the most specified state in patterning of the skeletogenic ectomesenchyme. The production of mice that ectopically express Otx2 or Hoxa-2 gene in this region is awaited. MORPHOGENESIS OF THE MAMMALIAN SKULL ACKNOWLEDGMENTS We wish to thank Dr. Noriko Osumi-Yamashita and Dr. Kazuhiro Eto for critical reading of the manuscript and for providing us with unpublished data. Our work cited was supported in part by grants-in-aid from the Ministry of Education, Science and Culture of Japan (Specially Promoted Research), and the Science and Technology Agency of Japan. REFERENCES Acampora, D., Mazan, S., Lallemand, Y., Avantaggiato, V., Maury, M., Simeone, A., and Brûlet, P. (1995) Forebrain and midbrain regions are deleted in Otx22/2 mutants due to a defective anterior neurectoderm specification during gastrulation. Development 121:3279–3290. Allin, E.F. (1975) Evolution of the mammalian middle ear. J. Morphol. 147:403–438. Allis, E.P. Jr. (1938) Concerning the development of the prechordal portion of the vertebrate head. J. Anat. 72:584–607. Andres, G. (1949) Untersuchungen an Chimären von Triton und Bombinator. I. Entwicklung xenoplastischer Labyrynthe und Kopfganglien. Genetica 24:387–534. Ang, S.-L., Conlon, R.A., Jin, O., and Rossant, J. (1994) Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development 120:2979–2989. Ang, S.-L., Jin, O., Rinn, M., Daigle, N., Stevenson, L., and Rossant, J. (1996) A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development 122:243–252. Balling, R., Mutter, G., Gruss, P., and Kessel, M. (1989) Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell 58:337–347. de Beer, G.R. (1931) On the nature of the trabecula cranii. Quart. J. microsc. Sci. 74:701–731. de Beer, G.R. (1937) ‘‘The Development of the Vertebrate Skull.’’ Oxford: Oxford University Press. Bellairs, A.D’A. (1958) The early development of the interorbital septum and the fate of the anterior orbital cartilages in birds. J. Embryol. Exp. Morphol. 6:68–85. Boncinelli, E., Gulisano, M., and Broccoli, V. (1993) Emx and Otx homeobox genes in the developing mouse brain. J. Neurobiology 24:1356–1366. Burke, A.C., Nelson, C.E., Morgan, B.A., and Tabin, C. (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121:333–346. Charité, J., Graaff, W. de and Deschamps, J. (1995) Specification of multiple vertebral identities by ectopically expressed Hoxb-8. Dev. Dyn. 204:13–21. Chisaka, O., and Capecchi, M.R. (1991) Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene Hox 1.5. Nature 350:473–479. Chisaka, O., Muski, T.S., and Capecchi, M.R. (1992) Developmental defects of the ear, cranial nerves and hindbrain resulting from targeted disruption of the mouse homeobox gene Hox-1.6. Nature 355:516–520. Cho, K.W.Y., Blumberg, B., Steinbeisser, H., and De Robertis, E.M. (1991) Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell 67:1111–1120. Cohen, S.M., and Jürgens, G. (1990) Mediation of Drosophila head development by gap-like segmentation genes. Nature 346:482–485. Condie, B.G., and Capecchi, M.R. (1994) Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature 370:304–307. Couly, G.F., Colty, P.M., and LeDouarin, N.M. (1993) The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 114:1–15. Couly, G., Grapin-Botton, A., Coltey, P., and Le Douarin, N.M. (1996) The regeneration of the cephalic neural crest, a problem revisited: The regenerating cells originate from the contralateral or from the anterior and posterior neural fold. Development 122:3393–3407. 153 Cserjesi, P., Lilly, B., Bryson, L., Wang, Y., Sasoon, D.A., and Olson, E.N. (1992) MHox: A mesodermally restricted homeodomain protein that binds an essential site in the muscle creatine kinase enhancer. Development 115:1087–1101. Dietrich, S., and Gruss, P. (1995) undulated phenotypes suggest a role of Pax-1 for the development of vertebral and extravertebral structures. Dev. Biol. 167:529–548. Finkelstein, R., and Perrimon, N. (1990) The orthodenticle gene is regulated by bicoid and torso and specifies Drosophila head development. Nature 346:485–488. Frasch, M., Chen, X., and Lufkin, T. (1995) Evolutionary-concerved enhancers direct region-specific expression of the murine Hoxa-1 and Hoxa-2 loci in both mice and Drosophila. Development 121:957– 974. Fürbringer, M. (1897) Über die spino-occipitalen Nerven der Selachier und Holocephalen ind ihre vergleichende Morphologie. Festschr. für Carl Gegenbaur. 3:349–788. Gaunt, S.J., Blum, M., and Robertis, E.M.D. (1993) Expression of the mouse goosecoid gene during mid-embryogenesis may mark mesenchymal lineages in the developing head, limbs, and body wall. Development 117:769–778. Gaupp, E. (1898) Die Metamerie des Schädels. Erg. Anat. Ent.-Ges. 7:793–885. Gaupp, E. (1902) Über die Ala temporalis des Säugerschädels und die Regio orbitalis einiger anderer Wirbeltierschädels. Anat. Heft. 15:433–595. Gaupp, E. (1912) Die Reichertsche Theorie. Arch. Anat. Phys. Suppl. 1912:1–416. Gegenbaur, C. (1898) ‘‘Vergleichende Anatomie der Wirbeltihiere mit Berücksichtung der Wirbellosen.’’ Leipzig: Verlag von Wilhelm Engelmann. Gendron-Maguire, M., Mallo, M., Zhang, M., and Gridley, T. (1993) Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 75:1317–1331. Goethe, J.W. (1790) Das Schädelgerüt aus sechs Wirbelknochen aufgebaut. Zur Naturwissenschaft Überhaupt, besonders zur Morphologie. II. 2. cited in Gaupp (1898). Goodrich, E.S. (1930) ‘‘Structure and Development of Vertebrates.’’ London: Macmillan. Graham, A., Heyman, I., and Lumsden, A. (1993) Even-numbered rhombomeres control the apoptotic elimination of neural crest cells from odd-numbered rhombomeres in the chick hindbrain. Development 119:233–245. Graham, A., Francis-West, P., Brickell, P., and Lumsden, A. (1994) The signalling molecule BMP-4 mediates apoptosis in the rhombencephalic neural crest. Nature 372:684–686. Hall, B.K., and Hörstadius, S. (1988) ‘‘The Neural Crest.’’ New York: Oxford University Press. Hanson, J.R., Anson, B.J., and Strickland, E.M. (1962) Branchial sources of the auditory ossicles in man. Part II. Observations of embryonic stages from 7 mm to 28 mm (CR length). Arch. Otolaryng. 76:200–215. Hoeden, F. van der, Sordino, P., Fraudeau, N., Izpisúa-Belmonte, J.-C., and Duboule, D. (1996) Teleost HoxD and HoxA genes: Comparison with tetrapods and functional evolution of the HOXD complex. Mech. Dev. 54:9–21. Holland, P.W.H., Garcia-Fernàndez, J., Williams, N.A., and Sidow, A. (1994) Gene duplications and the origins of vertebrate development. Development 1994 Suppl.:125–133. Holland, P.W.H., and Garcia-Fernàndez, J. (1996) Hox genes and chordate evolution. Dev. Biol. 173:382–395. Holmgren, N., and Stensiö, E. (1936) Kranium und Visceralskelett der Akranier, Cyclostomen und Fische. In: ‘‘Handbuch der vergleichenden Anatomie der Wirbeltiere,’’ Bolk, L., Göppert, E., Kallius, E., and Lubosch, W. (eds). Berlin und Wien: Urban & Schwarzenberg, vol. 4, pp. 233–499. Horan, G.S.B., Ramı̂rez-Soris, R., Featherstone, M.S., Wolgemuth, D.J., Bradley, A., and Behringer, R. (1995) Compound mutants for the paralogous hoxa-4, hoxb-4, and hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes Dev. 9:1667–1677. Hunt, P., Whiting, J., Muchamore, I., Marshall, H., and Krumlauf, R. 154 KURATANI ET AL. (1991) Homeobox genes and models for patterning the hindbrain and branchial arches. Development Suppl. 1:187–196. Hunt, P., Ferretti, P., Krumlauf, R., and Thorogood, P. (1995) Restoration of normal Hox code and branchial arch morphogenesis after extensive deletion of hindbrain neural crest. Dev. Biol. 168:584–597. Huxley, T.H. (1874) On the structure of the skull and of the heart of Menobranchus lateralis. Proc. Zool. Soc. London 1874 (cited by de Beer 1931). Imai, H., Osumi-Yamashita, N., Ninomiya, Y., and Eto, K. (1996) Contribution of early-emigrating midbrain crest cells to the dental mesenchyme of the mandibular molar tooth in rat embryos. Dev. Biol. 176:151–165. Janvier, P. (1993) Patterns of diversity in the skull of jawless fishes. In: ‘‘The Skull,’’ Hanken, J., and Hall, B.K. (eds). Chicago and London: University Chicago Press, vol. 2, pp. 131–188. Jarvik, E. (1980) ‘‘Basic Structure and Evolution of Vertebrates.’’ London: Academic Press. Jegalian, B.G., and De Robertis, E. (1992) Homeotic transformations in the mouse induced by overexpression of a human Hox3.3 transgene. Cell 71:901–910. Kastschenko, N. (1887) Das Schlundspaltengebiet des Hühnchens. Arch. Anat. Physiol. 1887:258–300. Kaufman, M.H., Chang, H.-H., and Shaw, J.P. (1995) Craniofacial abnormalities in homozygous Small eye (Sey/Sey) embryos and newborn mice. J. Anat. 186:607–617. Kemp, T.S. (1982) ‘‘Mammal-like Reptiles and the Origin of Mammals.’’ London: Academic Press. Kermack, D.M., and Kermack, K.A. (1984) ‘‘The Evolution of the Mammalian Characters.’’ London, Sydney: Croom Helm. Kessel, M. (1992) Respecification of vertebral identities by retinoic acid. Development 115:487–501. Kessel, M., and Gruss, P. (1991) Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67:89–104. Köntges, G., and Lumsden, A. (1996) Phombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122:3229–3242. Krumlauf, R. (1993) Hox genes and pattern formation in the branchial region of the vertebrate head. Trend. Genet. 9:106–112. Kuratani, S. (1989) Development of the orbital region in the chondrocranium of Caretta caretta. Reconsideration of the vertebrate neurocranium configuration. Anat. Anz. 169:335–349. Kuratani, S.C., and Wall, N.A. (1992) Expression of Hox 2.1 protein in restricted populations of neural crest cells and pharyngeal ectoderm. Dev. Dyn. 195:15–28. Kuratani, S.C., and Eichele, G. (1993) Rhombomere transplantation repatterns the segmental organization of cranial nerves and reveals cell-autonomous expression of a homeodomain protein. Development 117:105–117. Kuratani, S., Martin, J.F., Wawersik, S., Lillie, B., Eichele, G., and Olson, E. (1994) The expression pattern of the chick homeobox gene gMHox suggests a role in patterning of the limb and face and in compartmentalization of somites. Dev. Biol. 161:357–369. Kurihara, Y., Kurihara, H., Suzuki, H., Kodama, T., Maemura, K., Nagai, R., Oda, H., Kuwaki, T., Cao, W.-H., Kamada, N., Jishage, K., Ouchi, Y., Azuma, S., Toyoda, Y., Ishikawa, T., Kumada, M., and Yazaki, Y. (1994) Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 368:703–710. Lacalli, T.C., Holland, N.D., and West, J.E. (1994) Landmarks in the anterior central nervous system of amphioxus larvae. Phil. Trans. R. Soc. Lond. B 344:165–185. Le Lièvre, C.S. (1974) Rôle des cellules mesectodermiques issues des crêtes neurales céphaliques dans la formation des arcs branchiaux et du skelette viscéral. J. Embryol. Exp. Morphol. 31:453–577. Le Lièvre, C.S. (1978) Participation of neural crest-derived cells in the genesis of the skull in birds. J. Embryol. Exp. Morphol. 47:17–37. Le Lièvre, C.S., and Le Douarin, N.M. (1975) Mesenchymal derivatives of the neural crest: analysis of chimeric quail and chick embryos. J. Embryol. Exp. Morphol. 34:125–154. Le Mouellic, H., Lallemand, Y., and Brulet, P. (1992) Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell 69:251–264. Lohnes, D., Mark, M., Mendelsohn, C., Dollé, Dierich, A., Gorry, P., Gansmuller, A., and Chambon, P. (1994) Function of retinoic acid receptors (RARs) during development. (I) Craniofacial and skeletal abnormalities in RAR double mutants. Development 120:2723–2748. Lufkin, T., Dierich, A., LeMeur, M., Mark, M., and Chambon, P. (1991) Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell 66:1105–1119. Lufkin, T., Mark, M., Hart, C.P., Dollé, P., LeMeur, M., and Chambon, P. (1992) Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature 359:835–841. Lumsden, A., Sprawson, N., and Graham, A. (1991) Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development 113:1281–1291. Luo, J., Pasceri, P., Conlon, R.A., Rossant, J., and Giguère, V. (1995) Mice lacking all isoforms of retinoic acid receptor b develop normally and are susceptible to the teratogenic effects of retinoic acid. Mech. Dev. 53:61–71. MacKenzie, A., Ferguson, M.W.J., and Sharpe, P.T. (1991) Hox-7 expression during murine craniofacial development. Development 113:601–611. Mallo, M., and Gridley, T. (1996) Development of the mammalian ear: coordinate regulation of formation of the tympanic ring and the external acoustic meatus. Development 122:173–179. Manak, J.R., and Scott, M.P. (1994) A class act: Conservation of homeodomain protein functions. Development 1994 Suppl.:61–71. Manley, N.R., and Capecchi, M.R. (1995) The role of Hoxa-3 in mouse thymus and thyroid development. Development 121:1989–2003. Mansouri, A., Stoykova, A., Torres, M., and Gruss, P. (1996) Dysgenesis of cephalic neural crest derivatives in Pax72/2 mutant mice. Development 122:831–838. Mark, M., Rijli, F.M., and Chambon, P. (1995) Alteration of Hox gene expression in the branchial region of the head causes homeotic transformations, hindbrain segmentation defects and atavistic changes. Semin. Dev. Biol. 6:275–284. Marshall, H., Nonchev, S., Sham, M.H., Lumsden, A., and Krumlauf, R. (1992) Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into 4/5 identity. Nature 360:737– 741. Martin, J., Bradley, A., and Olson, E. (1995) The paired-like homeobox gene MHox is required for early events of skeletogenesis in multiple lineages. Genes Dev. 9:1237–1249. Matsuo, I., Kuratani, S., Kimura, C., Takeda, N., and Aizawa, S. (1995) Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev. 9:2646–2658. Matzuk, M.M., Kumar, T.R., Vassalli, A., Bickenbach, J.R., Roop, D.R., Jaenisch, R., and Bradley, A. (1995) Functional analysis of activin during mammalian development. Nature 374:354–357. McGinnis, W., and Krumlauf, R. (1992) Homeobox genes and axial patterning. Cell 68:283–302. McLain, K., Schreiner, C., Yager, K.L., Stock, J.L., and Potter, S.S. (1992) Ectopic expression of Hox-2.3 induces craniofacial and skeletal malformations in transgenic mice. Mech. Dev. 39:3–16. Mendelsohn, C., Lohnes, D., Décimo, D., Lufkin, T., LeMeur, M., Chambon, P., and Mark, M. (1994) Function of retinoic acid receptors (RARs) during development. (I) Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 120:2749–2771. Moore, W.J. (1981) ‘‘The Mammalian Skull.’’ London: Cambridge University Press. Morrison, A., Chaudhuri, C., Ariza-McNaughton, L., Muchamore, I., Kuroiwa, A., and Krumlauf, R. (1995) Comparative analysis of chicken Hoxb-4 regulation in transgenic mice. Mech. Dev. 53:47–59. Niederländer, C., and Lumsden, A. (1996) Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development 122:2367–2374. Noden, D.M. (1978) The control of avian cephalic neural crest cytodifferentiation. I. skeletal and connective tissues. Dev. Biol. 67:296–312. Noden, D.M. (1983a) The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev. Biol. 96:144– 165. Noden, D.M. (1983b) The embryonic origins of avian craniofacial muscles and associated connective tissues. Am. J. Anat. 186:257–276. MORPHOGENESIS OF THE MAMMALIAN SKULL Noden, D.M. (1988) Interactions and fates of avian craniofacial mesenchyme. Development 103 Suppl.:121–140. Osumi-Yamashita, N., Ninomiya, Y., Doi, H., and Eto, K. (1994) The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of mouse embryos. Dev. Biol. 164:409–419. Osumi-Yamashita, N., Ninomiya, Y., Doi, H., and Eto, K. (1996) Rhombomere formation and hindbrain crest cell migration from prorhombomeric origins in mouse embryos. Dev. Growth Differ. 38:107–118. Osumi-Yamashita, N., Kuratani, S., Ninomiya, Y., Aoki, K., Iseki, S., Chareonvit, S., Doi, H., Fujiwara, M., Watanabe, T., and Eto, K. (1997 ) Cranial anomaly of homozygous rSey rat is associated with a defect in the migration pathway of midbrain crest cells. Dev. Growth Differ. 39:53–67. Owen, R. (1866) ‘‘On the Anatomy of Vertebrates.’’ London: Longmans Green & Co. Pollock, R.A., Gilbert, J., and Bieberich, C.J. (1992) Altering the boundaries of Hox3.1 expression: Evidence for antipodal gene regulation. Cell 71:911–923. Porteus, M.H., Bulfone, A., Ciaranello, R.D., and Rubenstein, J.L.R. (1991) Isolation and characterization of a novel cDNA clone encoding a homeo domain that is developmentally regulated in the ventral forebrain. Neuron 7:221–229. Portmann, A. (1976) ‘‘Einführung in die vergleichende Morphologie der Wirbeltiere.’’ 5th ed. Basel: Schwabe & Co. Pourquie, O., Coltey, M., Teillet, M.A., Ordahl, C., and Le Douarin, N.M. (1993) Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc. Natl. Acad. Sci. USA 90:5242–5246. Presley, R., and Steel, F.L.D. (1976) On the homology of the alisphenoid. J. Anat. 121:441–459. Price, M., Lemaistre, M., Pischetola, M., Lauro, R.D., and DuBoule, D. (1991) A mouse gene related to Distal-less shows a restricted expression in the developing forebrain. Neuron 8:241–255. Prince, V., and Lumsden, A. (1994) Hoxa-1 expression in normal and transposed rhombomeres: independent regulation in the neural tube and neural crest. Development 120:911–923. Qiu, M., Bulfone, A., Martines, S., Meneses, J.J., Shimamura, K., Pedersen, R.A., Rubenstein, J.L.R. (1995) Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 9:2523–2538. Rabl, C. (1887) Über das Gebiet des Nervus facialis. Anat. Anz. 2:219–227. Ramirez-Solis, R., Rivera-Pérez, J., Wallace, J.D., Wims, M., Zheng, H., and Bradley, A. (1993) Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell 73:279–294. Reichert, K.B. (1837) Über die Visceralbogen der Wirbelthiere im Allgemeinen und deren Metamorphosen bei den Vögeln und Säugethieren. Arch. Anat. Phys. Wiss. Med. 1837:120–220. Rathke, H. (1839) Entwckelungsgeschichte der Unke. Königsberg (cited by de Beer, 1931). Raven, C.P. (1931) Zur Entwicklung der Ganglienleiste. I. Kinematik der ganglienleisten Entwicklung bei der Urodelen. Willhelm Roux’ Arch. Entwicklungsmech. Org. 125:210–292. Rijli, F.M., Mark, M., Lakkaraju, S., Dierich, A., Dollé, P., and Chambon, P. (1993) A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75:1333–1349. Rivera-Peréz, J.A., Mallo, M., Gendron-Maguire, M., Gridley, T., and Behringer, R.R. (1995) goosecoid is not an essential component of the mouse gastrula organizer but is required for craniofacial and rib development. Development 121:3005–3012. Robinson, G.W., and Mahon, K.A. (1994) Differential and overlapping expression domains of Dlx-2 and Dlx-3 suggest distinct rolesfor Distal-less homeobox genes in craniofacial development. Mech. Dev. 48:199–215. Robinson, G.W., Wray, S., and Mahon, K.A. (1991) Spatially restricted expression of a member of a new family of murine Distal-less homeobox genes in the developing forebrain. New Biol. 3:1183–1194. 155 Saldivar, J.R., Krull, C.E., Krumlauf, R., Ariza-McNaughton, and Bronner-Fraser, M. (1996) Rhombomere of origin determines autonomous versus environmentally regulated expression of Hoxa 3 in the avian embryo. Development 122:895–904. Sarnat, H.B., and Netsky, M.G. (1981) ‘‘Evolution of the Nervous System.’’ 2nd ed. New York, Oxford: Oxford University Press. Satokata, I., and Maas, R. (1994) Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nature Genet. 6:348–356. Schorle, H., Meier, P., Buchert, M., Jaenisch, R., and Mitchell, P.J. (1996) Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature 381:235–238. Sechrist, J., Serbedzija, G.N., Sherson, T., Fraser, S., and BronnerFraser, M. (1993) Segmental migration of the hindbrain neural crest does not arise from segmental generation. Development 118:691–703. Shawlot, W., and Behringer, R.R. (1995) Requirement for Lim1 in head-organizer function. Nature 374:425–430. Shigetani, Y., Aizawa, S., and Kuratani, S. (1995) Overlapping origins of pharyngeal arch crest cells on the postotic hindbrain. Dev. Growth Differ. 37:733–746. Simeone, A., Acampora, D., Gulisano, M., Stornaiuolo, A., and Boncinelli, E. (1992) Nested expression domains of four homeobox genes in developing rostral brain. Nature 346:763–765. Slack, J.M.W., Holland, P.W.H., and Graham, C.F. (1993) The zootype and the phylotypic stage. Nature 361:490–492. Stadmüller, F. (1936) Kranium und Visceralskelett der Säugetiere. In: ‘‘Handbuch der vergleichenden Anatomie der Wirbeltiere,’’ Bolk, L., Göppert, E., Kallius, E., and Lubosch, W. (eds). Berlin und Wien: Urban & Schwarzenberg. vol. 4, pp. 839–1016. Starck, D. (1979) ‘‘Vergleichende Anatomie der Wirbeltiere auf evolutionsbiologischer Grundlage.’’ Berlin, Heidelberg, New York: Springer. Stone, L.S. (1926) Further experiments on the extirpation and transplantation of mesectoderm in Amblystoma. J. Exp. Zool. 44:95–131. Strickland, E.M., Hanson, J.R., and Anson, B. (1962) Branchial sources of auditory ossicles in man. I. Literature. Arch. Otolaryngol. 76:100–122. Suda, Y., Matsuo, I., Kuratani, S., and Aizawa, S. (1996) Otx1 function overlaps with Otx2 in development of forebrain and midbrain. Genes to Cells 1:1031–1044. Suemori, H., Takahashi, N., and Noguchi, S. (1995) Hoxc-9 mutant mice show anterior transformation of the vertebrae and malformation of the sternum and ribs. Mech. Dev. 51:265–273. Thorogood, P. (1988) The developmental specification of vertebrate skull. Development 103:141–153. Torrey, T.W., and Feduccia, A. (1979) ‘‘Morphogenesis of the Vertebrates.’’ 4th ed. New York: John Wiley & Sons. von Baer, K.E. (1828) ’’Entwicklungsgeschichte der Thiere: Beobachtung und Reflexion.’’ Born Träger, Königsberg. Wagner, G. (1949) Die Bedeutung der Neuralleiste für die Kopfgespaltung der Amphibienlarven. Rev. Swisse Zool. 56:519–620. Wagner, G. (1959) Untersuchungen an Bombinator-Triton-Chimaeren. Roux’s Arch. Ent. Mech. Org. 151:36–158. Williams and Warwick (1990) ‘‘Gray’s Anatomy.’’ 36th ed. London: Churchill Livingstone. Winslow, G.M. (1898) The chondrocranium in the Ichthyopsida. Tufts Coll. Stud. 5:147 (cited in de Beer, G.R., 1937). Yamada, G., Mansouri, M., Terres, M., Blum, M., Stuart, E.T., Schultz, M., De Robertis, E.M., and Gruss, P. (1995) Targeted mutation of the mouse goosecoid gene results in craniofacial defects and neonatal death. Development 121:2917–2922. Yoshida, M., Suda, Y., Takeda, N., Matsuo, I., Kuratani, S., and Aizawa, S. (1997) Developmental function of Emx genes in overlapping domains of the forebrain. Development 124:101–111. Zhang, J., Hagopian-Donaldson, S., Serbedzija, G., Elsemore, J., Plehn-Dujowich, D., McMahon, A.P., Flavell, R.A., and Williams, T. (1996) Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 381:238–241. Zhang, M., Kim, H.-J., Marshall, H., Gendron-Maguire, M., Lucas, D.A., Baron, A., Gudas, L.J., Gridley, T., Krumlauf, R., and Grippo, J.P. (1994) Ectopic Hoxa-1 induces rhombomere transformation in mouse hindbrain. Development 120:2431–2442.