* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Oxidative phosphorylation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Signal transduction wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Transcript
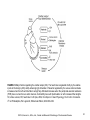
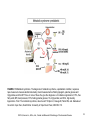
Chapter 2 Cardiac Metabolism in Health and Disease © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease FIGURE 2.1 How carbohydrates and fatty acids yield energy for contractile work. Crucial are the processes whereby the fuels (glucose and fatty acids) are simplified for entry into the Krebs cycle that lies within the mitochondria. Their production of protons (H) within yields energy in the form of ATP (adenosine triphosphate) that is mostly used for contractile work. © LH Opie, 2004, from Opie LH. Heart Physiology, From Cell to Circulation. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:308–354. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 2 FIGURE 2.2 The ATP–ADP cycle. Energy in the form of ATP is produced by the Krebs citrate cycle (Fig. 2.3). Heart work utilizes the high energy liberated from ATP in the conversion to ADP which is then resynthesized together with inorganic phosphate (Pi) to produce ATP by oxidative phosphorylation. © LH Opie, 2004, from Opie LH. Heart Physiology, From Cell to Circulation. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:308–354. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 3 FIGURE 2.3 Glycolysis simplified. G-6-P, glucose 6-phosphate; PFK, phosphofructokinase; F 1,6 bisP, fructose 1,6 bisphophate. © LH Opie, 2004, from Opie LH. Heart Physiology, From Cell to Circulation. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:308–354. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 4 FIGURE 2.4 The citrate cycle of Krebs. In reality, the following reactions are readily reversible: citrate → isocitrate; succinate → oxaloacetate via the intervening reactions. The most important potential sites of control are citrate synthase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and malate dehydrogenase (by regulating the supply of oxaloacetate). Of these, isocitric dehydrogenase and α-ketoglutarate dehydrogenase are calciumsensitive, as is pyruvate dehydrogenase (PDH). These dehydrogenases respond to increased cytosolic calcium (as in inotropic stimulation) by increased activity. © LH Opie, 1998. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 5 FIGURE 2.5 Substrate metabolism in the fasted state. Blood levels of free fatty acids are high in the fasted state or poorly controlled diabetes mellitus. High levels of blood free fatty acids are oxidized by the heart in preference to glucose and lactate. Use of lipid accounts for 60–70% of the oxygen uptake of the heart, whereas use of carbohydrate accounts for less than 20%. © LH Opie, 2004, from Opie LH. Heart Physiology, From Cell to Circulation. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:308–354. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 6 FIGURE 2.6 Substrate metabolism in the fed state. After a high carbohydrate meal or glucose feeding, blood glucose and insulin are high, and blood free fatty acids low. Glucose becomes the major fuel of the heart, and carbohydrate can account for 50–75% of the oxygen uptake. CHO, carbohydrate. © LH Opie, 2004, from Opie LH. Heart Physiology, From Cell to Circulation. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:308–354. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 7 FIGURE 2.7 Effects of excess FFA in muscle cells. Molecular steps that lead from increased circulating FFA to insulin resistance (top left) exist together with opposing influences exerted by exercise or the antidiabetic drug metformin (top left and top right). Excess FFA entering the cell is activated to long-chain acyl-CoA, which inhibits the insulin signaling pathway. Thus there is less translocation of glucose transporter vesicles (GLUT-4 and -1) to the cell surface. The result is that muscle glucose uptake is decreased and hyperglycemia is promoted. Exercise and metformin, by stimulating the enzyme adenosine monophosphate-activated kinase (AMPK), promote the translocation of transport vesicles to the cell surface to promote glucose entry and thereby oppose insulin resistance. Protein kinase B, also called Akt, plays a key role. IRS-P, insulin receptor substrate-1; P-I-3 kinase, phosphatidyl inositol-3-kinase; PKB, protein kinase B. © LH Opie, 2009. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 8 FIGURE 2.8 Long-chain free fatty acid (FFA) metabolism. Effects of excess FFA supply. Blood borne FFA is taken into the cytosol, where the acyl carnitine carrier on the mitochondrial membrane transfers activated fatty acid (acyl CoA) into the mitochondrial space for oxidation in the fatty acid oxidation cycle. ACS/HF, acute coronary syndrome, heart failure; acyl CoA, long-chain acyl CoA compounds; CPT, carnitine palmityl CoA transferase; acyl carnitine, long-chain acyl carnitine compounds; CAT, carnitine acyl translocase; FFA, free fatty acids; GIK, glucose-insulin- potassium; MM, mitochondrial PDH, pyruvate dehydrogenase. © LH Opie, 2004, from Opie LH. Heart Physiology, From Cell to Circulation. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:308–354. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 9 FIGURE 2.9 Major factors regulating the cardiac output (CO). The heart rate is regulated chiefly by the relative inputs of cholinergic (ACh) and β-adrenergic (β) stimulation. Preload is regulated by the venous return and also increases when the left ventricle fails to empty fully. Afterload increases when the peripheral vascular resistance (PVR) rises or when there is aortic stenosis. Contractility rises with β-stimulation or with increased fiber lengths. SV, stroke volume; HR, heart rate. © LH Opie, 2004, from Opie LH. Heart Physiology, From Cell to Circulation. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:308–354. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 10 FIGURE 2.10 Beta-adrenergic signaling. β-Adrenergic signal systems involved in positive inotropic and lusitropic (enhanced relaxation) effects. © LH Opie, 2012, from Opie LH, Gersh BG (editors) Drugs for the Heart, 8th edition, Elsevier, Philadelphia, 2013. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 11 FIGURE 2.11 Metabolic cardioprotection during acute coronary syndromes. Critical is reduction of circulating free fatty acids (FFA) both by beta-blockade and by infusion of glucose-insulin-potassium (GIK). Thereby protective glycolytic metabolism by the ischemic tissue is promoted. The harm of FFA is exerted by increasing tissue fatty acid acyl CoA and carnitine, thereby promoting membrane damage and lethal arrhythmias. TG = tissue triglycerides. © LH Opie, 2004, from Opie LH. Heart Physiology, From Cell to Circulation. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2004:308–354. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 12 FIGURE 2.12 Metabolic syndrome. The diagnosis of metabolic syndrome, a prediabetic condition, requires a tape measure to measure abdominal obesity, blood measurements of fasting lipogram, plasma glucose and triglycerides, and the BP. Three or more of these five give the diagnosis. A-II indicates angiotensin-II; FFA, free fatty acids; BP, blood pressure; FPG, fasting plasma glucose; TG, triglycerides; and HDL, high-density lipoproteins. From: The metabolic syndrome, does it exist? © Opie LH, Kasuga M, Yellon DM, eds. Diabetes at the Limits. Cape Town, South Africa: University of Cape Town Press; 2006:95–110. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 13 FIGURE 2.13 Defects of energy transfer in HF. Note depressed activity of creatine kinase (CK) and depressed flux of phosphocreatine (PCr) through CK. The decreased ATP supply impairs the uptake of Ca 2+ ions into the sarcoplasmic reticulum (SR), thereby leading to decreased contractile force. ATP located near the cell membrane is also decreased thus depressing Na+ pump activity which in turn leads to an increased cytosolic Na+ and impaired Na+ /K+ exchange. The overall result of the deficits in the energy supply is the decreased contractile force characteristic of the failing myocardium. CK, creatine kinase; PCr, phosphocreatine. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 14 FIGURE 2.14 FFA effects on mitochondria. High circulating levels of free fatty acids (FFA), activated within the muscle cell to fatty acid CoA, enter the mitochondria by the action of CPT-1, thereby inhibiting the action of pyruvate dehydrogenase (PDH), blocking protective glycolysis that supplies cytosolic ATP used for ion pumps, and decreasing glucose uptake by muscle. Excess activated FFA increases formation of ROS. As protons are passed along the electron transport chain, ROS are formed at complexes I and III. FFA-induced proton leakage passes through uncoupling proteins which divert protons from ATP formation and hence waste oxygen. CPT 1, carnitine palmityl CoA transferase; mPTP, mitochondrial permeability transition pore; ROS, reactive oxygen species. © 2014, Elsevier Inc., Willis, et.al., Cellular and Molecular Pathobiology of Cardiovascular Disease 15