* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download THE VARIATION O F .THE STRESS OPTICAL COEFFICIENT WITH
Survey
Document related concepts
Atmospheric optics wikipedia , lookup
Nonimaging optics wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Photon scanning microscopy wikipedia , lookup
Silicon photonics wikipedia , lookup
Optical tweezers wikipedia , lookup
3D optical data storage wikipedia , lookup
Optical coherence tomography wikipedia , lookup
Optical aberration wikipedia , lookup
Ellipsometry wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Nonlinear optics wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Retroreflector wikipedia , lookup
Smart glass wikipedia , lookup
Transcript
THE VARIATION O F .THE STRESS OPTICAL COEFFICIENT
WITH GLASS COMPOSITION
by
Tod Renard Nissle
A Thesis Submitted to the Faculty of the
DEPARTMENT OF METALLURGICAL ENGINEERING
In Partial Fulfillment of the Requirements
For the Degree of
MASTER OF SCIENCE
WITH A MAJOR IN MATERIALS ENGINEERING.
In the Graduate College
THE UNIVERSITY OF ARIZONA
1 9 7 .3
STATEMENT BY AUTHOR
This thesis has been submitted in partial fulfillment of
requirements for an advanced degree at The University of Arizona and
is deposited in the University Library to be made available to borrowers
under rules of the Library.
Brief quotations from this thesis are allowable without special
permission, provided that accurate acknowledgment of source is made.
Requests for permission for extended quotation from or reproduction of
this manuscript in whole or in part may be granted by the head of the
major department or the Dean of the Graduate College when in his judg
ment the proposed use of the material is in the interests of scholar
ship.
In all other instances, however, permission must be obtained
from the author.
SIGNED:
APPROVAL BY THESIS DIRECTOR
This thesis has been approved on the date shown below:
WALTER W. WALKER
Associate Professor of
Metallurgical Engineering
Date
ACKNOWLEDGMENTS
Since they are responsible for the completion of this
thesis, the author must give the inadequate reward of appearing
on this page to:
Mr. Warren Turner for his unerring explanations,
apparatus innovations and otherwise; Mr. Roger Johnston, the master
glassmaker; Dr. Walter W. Walker, Associate Professor of Metal
lurgical Engineering at The University of Arizona, for his patience
and aide in the dull task of finalizing the thesis; and Dr.
Clarence L. Babcock, Professor of Optical Sciences at The University
of Arizona, who, whenever the author began to stray, quietly
appeared on the scene to point the way.
This investigation was sponsored under Project THEMIS,
administered by the United States Air Force Office of Scientific
Research under Contract No. F44620-69-C-0024.
TABLE OF CONTENTS
Page
LIST OF TABLES
ABSTRACT
........
. . . . . .
. . . . .
.....................................
............
i
INTRODUCTION
. . . . . . . . . . . . . . . . . . . . . . .
LITERATURE REVIEW
.......................................
2.1
2.2
.
2
Bartholinus Discovers Birefringence
............
The Index of Refraction as a Measure of
Birefringence
...................
..........
2.3
Huygens’ Misconception of Light Waves ...
2.4
The Nature of L i g h t ..........
2.5
Polarized Light and Birefringent Material
. . . . . .
2.6
The Photoelasticity of Glass
2.7
The Theory of P h o t o e l a s t i c i t y ..........
2.8
The Variation of Birefringence with Composition
.. .
2;9
Application of Data on the Variation of the Stress
Optical Coefficient with Composition
,
2.10 In Review
. . . . . . . .
:■ 3
. . . . . . x
xiii
CHAPTER
1
vii
.
LIST OF ILLUSTRATIONS
PREPARATION OF GLASS SAMPLES USED TO MEASURE
BIREFRINGENCE
. . . . . . . . . . . . . . . . . . . . .
3.1
3.2
3.3
3.4
3.5
3.6
3.7
3.8
3.9
1
. . . . . . .
5
5
7
11
17
24
28
31
33
39
40
Weighing of Glass Constituents ... . . . . . . .. . .
41
Mixing of Glass Constituents .
.
41
Melting the Batch
..............
41
Quenching, Washing and Drying the Glass . . . . . . .
47
Remelting and Stirring the Glass . . . . . .
........
48
Pouring the Glass
.
.......... .. . . .
.........
48
Annealing the Glass Disk . . . . . . :. . . .. . . . . .
48
Residual Strain and Compositional Uniformity- . . . . . . 49
Overall Chemical Composition of the Glass
. . . . . .
50
3.9.1 Procedure Followed in Measuring the Refractive
Index of a G l a s s ............
51
3.9.2 Evaluation of Measured Refractive Indices . . . . 5 1
3.10 Cutting and Finishing of the Glass S a m p l e ....
54
3.11 The Finished Piece of Glass
. . . . . . . .
. . .. .
54
iv
V
TABLE OF CONTENTS— Continued
Page
4
OPTICAL SYSTEM AND MEASUREMENT P R O C E D U R E ..........
57
4.1
57
60
60
60
62
62
62
64
64
67
67
67
67
68
69
4.2
5
DATA
5.1
5.2
5.3
6
The Optical System
....................
4.1.1 Light Source . . . .
............
4.1.2
Mdnochrometer or First Lens
. . . . . . . . .
4.1.3
Interference Filter
. . . . .
...
4.1.4 Pinhole
....................... .
4.1.5
Second Lens
. . . . . . . . . . . . . . . . .
4.1.6
First Polarizer
.
4.1.7
Glass Specimen Under Uniaxial Stress . . . . .
4.1.8 Soleil-Babinet Compensator
.......
4.1.9 Second Polarizer or Analyzer . . . . . . . . .
4.1.10 Third L e n s .................
4.1.11 Photomultiplier and Oscilloscope . . . . . . .
Experimental Procedure. . . . . . . . .
. . . . . . .
4.2.1
Alignment
. . . . . .
4.2.2
Orientation of the Polarizers
. . . . . . . .
4.2.3
Measurement of the Retardation of the
Ordinary Behind the Extraordinary Ray . . .
ANALYSIS
. .
..........
6.1
6.2
80
....
Calculation of Stress Optical Coefficients
Statistical Analysis
. . . ............
5.2.1
Standard Deviation . . . . . . . . . . . . . .
5.2.2
Student's "t" Test
.
Linear Regression Analysis
. . . . . . . . .
. ...
RESULTS AND DISCUSSION . . . . . . . . .
.. . . .
70
...
.
Initial Investigation . . . . . . . .. . . . . .
..
Preparation of Glass Samples
.......... ... . . .
6.2.1 Melting
.
. . . .
6.2.2 Annealing
..........
6.2.3 Grinding and P o l i s h i n g ............
6.3
Measurement of the Stress Optical Coefficient
. . . .
. 6.4
Linear Regression Analysis
. . . . . . . .
.. . . .
6.4.1
Soda-Titania-Silica Glasses
. . . . . . . .
.
6.4.2 Soda-Alumina-Silica Glasses
... . . . . .
80
83
84
84
88
99
100
104
104
105
105
106
107
107
109
TABLE OF CONTENTS— Continued
Page
7
CONCLUSIONS
. . . .
. . . . . . . . .
I . .
CHEMICAL ANALYSIS .
APPENDIX B:
COMPUTER PROGRAM USED FOR LINEAR REGRESSION
ANALYSIS OF STRESS OPTIC D A T A ........ ..
117
EQUIPMENT EMPLOYED IN THE PREPARATION
SAMPLES
..........
124
APPENDIX D :
........
112
APPENDIX A:
APPENDIX C:
. . ...
. . . . . .
OFGLASS
. . . .
EQUIPMENT EMPLOYED IN MEASURING THE STRESS
OPTICAL COEFFICIENT OF A GLASS SAMPLE
. . . . .
SELECTED BIBLIOGRAPHY
114
128
134
LIST OF ILLUSTRATIONS
Figure
Page
1.
The double refraction of light by calcite .
.........
6
2.
The longitudinal vibration of sound waves . . . . . . . . .
8
3.
The transverse vibration of light waves .............
9
4.
The two perpendicular components, E and E , of the
electric vector, E
. . . . . . . . . . .
...
10
5.
The electric and magnetic Vectors of a light wave
. . . . .
6.
Two dimensional representation of the electric field lines
of an electron, e, .after Ernsberger (1970)
. . . . ... . . .
12
14
7.
Field of an oscillating electron after Ernsberger
8.
Reduction of the electric vectors of a light ray, L, to
one equivalent vector, E^ .
..........
16
Division of an incident beam of polarized light, L^, into
two wavefronts, L and L , upon entering birefringent
material
. . . .e . . . ? .
...................
18
10.
Linearly of plane polarized l i g h t .............
20
11.
Circularly polarized light
21
12.
Elliptically polarized light
. . . . . . .
22
13.
Linearly polarized light ray, L^, becoming ellipticaily
polarized by traversing a birefringent crystal
. . . . . .
23
Linearly polarized light ray, L^, passing through
isotropic glass . . . . . . . . . .
........
. . . . . . . .
25
Homogeneous glass acquiring the properties of a uniaxial
anisotropic crystal by being subjected to uniaxial
.
............ .
compression, P . . . . .
26
Deformation of a glass block under uniaxial
compression, P . . . . . . . . . . . . . . . .
27
9.
14.
15.
16.
\
. . . .. . .
vil
'
(1970). 1 5
. . . . . . . . . .
■
. . . . .
-
;■
viii
LIST OF ILLUSTRATIONS— Continued
Page
Figure
17.
18.
19.
20.
21.
22.
23.
Mueller concepts of "lattice effect" and "atomic
effect" for glass under compressive force, P, after
Ernsberger (1970)
... . . .
........ .. ... . . . .
. .
30
Comparison of Adams and Williamson's and Pockels' data of
birefringence vs. composition for flint glasses after
Adams and Williamson (1919)
.
. . . . . . .
32
Variation of stress optical coefficient, C, with glass
composition after Waxier and Napolitano (1957) . . . .
..
34
Linear variation of refractive index in phase fields of
the NagO-CaO-SiOg glass system after Babcock (1968)
. . .
36
Linear variation of Knoop hardness in phase fields of
the NagO-CaO^SiOg glass system after.Georoff (1972)
. .
37
Equations used to calculate data plotted in the N2S and
N3C6S phase fields in Figures 20 and 21 after Babcock
(1968) and Georoff (1972)
. .. .. . . . . . .
. . . ..
38
Preparation of a glass sample and important factors
affecting glass homogeneity
.................
.
..
42
Dimensions of the finished sample and criteria used
• in its preparation . . . . . .
, . ... . . . . . . .
..
55
25.
Procedure for preparing finished glass samples . . . .
..
56
26.
Optical bench arrangement
. . ..
58
27.
Continued optical bench arrangement
..
59
28.
Polarization of light traveling through the optical,
system . . . . . .. . . . . .
.. . . . . . . . . , . . ..
61
29.
Glan-Thompson polarizer
63
30.
The Soliel-Babinet compensator
24.
.
..
.. . . . . . . .
. . . . . . .
.
. . . .
. . . . .
. . . . . .
.. . . . . . .
. . . ..
.
66
Affect of the positioning of the Babinet compensator on
extinction points (y values) obtained . . . . . . . . . .
72
31.. Setting a Babihet compensator as a quarter wave plate
32.
65
.
ix
LIST OF ILLUSTRATIONS— Continued
Figure
33.
Page
Definition of the "b” and "d" dimensions of the
glass specimens . . . , . . . . . . . . . . . . .
... .
82
Stress optical coefficient, C, versus mole fraction of
aluminum oxide for the five commercial laboratory
melted glasses in the nepheline and corundum phase
fields of the soda-alumina-silica ternary system
... .
101
Index of refraction, N, versus mole fraction of aluminum
oxide for the five commercial laboratory melted glasses
in the nepheline and corundum phase fields of the sodaalumina-silica ternary system after Babcock (1968)
. . .
102
Knoop hardness number, K.H.N., versus mole fraction of
aluminum oxide for the five commercial laboratory melted
glasses in the nepheline and corundum phase fields of
the soda-alumina-silica ternary system after Georoff
(1972)
. . . . . . . . . . .
.. . . . . . . . ...
103
Lines of equal stress optical coefficient in primary
phase fields D. (unknown) and E (Na^O.TiOg.SiOg) of
the soda-titania-silica ternary system . . . . . . . . .
108
Linear variation of stress optical coefficient in the
nepheline phase field of the Na 2 0 -Al 2 0 2 _Si 0 2 glass
........... . . . . . . . . . . . '
system
. . . . . .
111
D-l.
Apparatus for aligning and compressing the glass sample .
131
D-2.
Detail of steel caps:
133
34.
. 35.
36.
37.
38.
(jT)
in Figure D-l . . . . . . . .
LIST OF. TABLES
Table
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
Page
Chemical Composition and Critical Temperatures for
Glasses Investigated in the D (Unknown) Phase Field
of the Soda-Titania-Silica Ternary System
............
43
Chemical Composition and Critical Temperatures for
Glasses Investigated in the E (Na„0•TiO^•Si02) Phase
Field of the Soda-Titania-Silica Ternary System
. . . . .
44
Chemical Composition of the Five Commercial Laboratory
. Melted Glasses in the Nepheline and Corundum Phase
Fields of the Soda-Alumina-Silica Ternary System .........
45
Chemical Composition and Critical Temperatures for
Glasses Investigated in the Nepheline Phase Field of the
Soda-Alumina-Silica Ternary System . . .
..........
46
Index of Refraction Values for Glasses in the D and E
Phase Fields of the Soda-Titania-Silica Ternary System . .
52
Index of Refraction Values for Commercial Laboratory
Melted Glasses in the Nepheline and Corundum Phase
Fields of the Soda-Alumina-Silica Ternary System . . . . .
53
Calibration of the Optical System through Measurement
of the Stress Optical Coefficient of National Bureau
of Standards (NBS) Sample BF 588
...................
74
Data Recorded in Measuring the Stress Optical Coefficient
of Soda-Alumina-Silica Glass A3
. . . . .
. .............
75
Data Obtained in Measuring the Stress Optical Coefficients
of Soda-Alumina-Silica Glasses
..........................
76
Data Obtained in Measuring the Stress Optical Coefficients
of Glasses in the D Field of the Soda-Titania-Silica
Ternary System . . . . . . . . . . . . . . . . . . . . . .
78
Data Obtained in Measuring the Stress Optical Coefficients
of Glasses in the E Field of the Soda-Titania-Silica
Ternary System .................
79
x
xi
LIST OF TABLES— Continued
Table
12.
13.
14.
.15.
16.
17.
18.
19V
.
20.
A-l.
B-l.
C-l.
Page
Statistical Data on Glasses in the Nepheline and
Corundum Phase Fields of the Soda-Alumina-Silica
Ternary System
.........
85
Statistical Data on Glasses in the D Phase Field of the
Soda-Titania-Silica Ternary System . . . . . . . . . . . . .
86
Statistical Data on Glasses in the E Phase Field of the
....................
Soda-Titania-Silica Ternary System .
87
Average Stress Optical Coefficient Values for Commercial
Laboratory Melted Glasses in the Nepheline and Corundum
Primary Phase Fields of the Soda-Alumina-Silica Ternary
.System
.......................... . . .
91
Average Stress Optical Coefficient Values for Glasses in
the Nepheline Primary Phase Field of the Soda-AluminaSilica Ternary System
. . . . . . ........................
93
Average Stress Optical Coefficient Values for Glasses in
the Nepheline and Corundum Primary Phase Fields of the
Soda-Alumina-Silica Ternary System ...........
.
94
Average Stress Optical Coefficient Values for Glasses in
the D Primary Phase Field of the Soda-Titania-Silica
Ternary System . . . . . . , . . . . . . .
.
95
Average Stress Optical Coefficient Values for Glasses in
the E Primary Phase Field of the Soda-Titania-Silica
Ternary System .
............
96
Average Stress Optical Coefficient Values for Glasses in
the D and E Primary Phase Fields of the Soda-TitaniaSilica Ternary System
.......................
97
Chemical Analysis of the Raw Glass Making Materials
Melted in this Investigation
............................
115
Computer Program Used for Linear Regression Analysis for
Glasses in the Sodium-Titanium-Silicate Ternary System . .
118
Equipment Employed in Preparing Glass Samples
125
.
. . . . .
xii
LIST OF T A B L E S - Continued
Table
D-l.
D-2.
Page
Equipment Employed in Measuring the Stress Optical
Coefficient of a Glass . . . . . . . . . . .
.........
Itemized Description of Apparatus in Figure D-l
. .
. . . . .
129
132
ABSTRACT
Refractive index, specific volume, Knoop hardness and fluidity
have a linear relationship with composition in primary phase fields.
This enables these properties to be computed from a glass' composition.
The stress optical coefficient, another important property of glass,
is used to evaluate a glass' residual strain.
The objective of this
study was to investigate whether the stress optical coefficient
(1) also varied linearly j.n primary phase fields and (2) could then be
determined from a glass' composition.
Glass specimens were prepared from the D and E phase fields
of the soda-titania-silica ternary system, and from the nepheline
and corundum phase fields of the soda-alumina-silica ternary system.
Each specimen, after being placed under uniaxial compression, then
had its stress optical coefficient measured.
The stress optic data
obtained were subjected to linear regression analysis to determine
whether they could accurately be described by the equation of a line.
The stress optical coefficient was found to vary linearly
with composition within primary crystallization phase, fields.
CHAPTER 1
INTRODUCTION
In 1669 Galileo pieced together a model of the telescope
invented by the Dutch lensmaker Jacoma (Halsey 1963).
Successive
generations have since, with varied success, strived to better
optical lens and mirrors.
.
A critical property of a lens or mirror
is residual stress.
When a glass is poured into a mold, the peripheral surface
solidifies first, the center last.
The outer crust is then in
compression while the central portion, which tried to contract on
cooling but was held by the solid crust, is under tension.
If the
glass is allowed to remain at and cool to room temperature after it
has solidified, the internal strains will often cause it to become
riddled with cracks and, at times, to explode into tiny fragments.
This is why a glass is put into an annealing furnace just after it
has solidified.
The furnace slowly decreases the temperature
and allows atoms to rearrange to a more stable configuration.
A
completely strain free glass is attained only by annealing for
infinite time.
At less than an infinite annealing, time, residual
strain always remains in the glass.
It should be noted that "residual strain" is actually .
residual stress in a material engineering sense; however, in
glass technology literature the two terms are used interchangeably.
This thesis will follow the glass technology terminology.
If residual tensile and compressive strain is high, a "flat"
surface cut across the glass will buckle and be uneven like a
rumpled shirt.
It is impossible to polish this surface to the
millionth of ah inch accuracy required on large telescope mirrors,—
for once one "rumple" is polished away another appears.
Since the
strain in a glass must be small before an accurate flat surface
m ay be obtained, it is necessary to be able to measure residual
strain.
So the question is, how may strain in glass be gauged?
Strain in glass is detected by measuring its effect on
polarized light or, in other words, by measuring its birefringence.
Birefringence is the difference between the refractive indices of
two plane-polarized light waves formed on traversing glass.
A glass
containing residual stress has a value of birefringence which is
usually greater than zero.
If the glass is isotropic (it has no
residual strain) the birefringence is zero.
However, isotropic glass
placed under stress as in Figure 15 becomes birefringent:
effect is called photoelasticity.
this
Brewster (1814) found that
birefringence was proportional to the strain in a glass block.
Birefringence thus serves as a direct measure of strain in glass.
While birefringence represents strain in glass, the stress
optical coefficient, a relation between the stress on and strain
of glass, is, as an extension of birefringence, also used to analyze
' residual strain.
The stress optical coefficient equals the
3
birefringence divided by the stress on the glass.
Lillie and Hitland
(1954) showed that the stress optical coefficient is needed to
develop annealing schedules that will keep the amount of birefringence
within prescribed limits.
Van Zee and Noritake (1958) and McGraw
and Babcock (1959) used the stress optical coefficient to determine
the rate of stress release over the annealing range of soda-lime,
potash-rbarium and borosilicate glasses.
Babcock (1968, 1969) demonstrated that certain physical
properties vary linearly in primary phase fields and so can be
accurately predicted by computer if the composition of the glass
is known.
Data on stress optical coefficients is scattered throughout
the literature, but it has not been determined whether birefringence
varies linearly in primary phase fields.
Since the stress optical
coefficient reflects the degree of change of a glass' physical
properties: when it is stressed, being: able to obtain the stress
optical coefficient from a computer program, with the glass
composition as input, would be handy.
if this can be done.
This study will determine
CHAPTER 2
LITERATURE REVIEW
. Since the first observation of the phenomenon of birefringence
in 1669 there have been many studies examining its occurence and
application.
The purpose of this paper is to continue the investi
gation by studying whether birefringence varies linearly with glass
composition.
As foundation to the study, it is desirable to review
the evolution of birefringence.
In recording this information an
attempt was made to mesh historical developments with the theoretical
background employed in analyzing.birefringence; hence, the following
will briefly be considered:
2.1
Bartholinus Discovers Birefringence.
2.2
The Index of Refraction as a Measure of Birefringence
2.3
Huygens' Misconception of Light.Waves
2.4
The Nature of Light
2.5
Polarized Light and Birefringent Material
2.6
The Photoelasticity of Glass
2.7
The Theory of Photoelasticity
2.8
The Variation of Birefringence with Composition
2.9
Application of Data on the Variation of the Stress
Optical Coefficient with Composition
2.10
In Review
2.1
Bartholinus Discovers Birefringence
Erasmus Bartholinus, a Scandinavian physicist, mathematician
and physician, was known for his observation and illustration of
snow crystals.
In 1669 he discovered what Huygens (Halsey 1963)
called the "strange refraction" of Iceland spar, a type of calcite.
Bartholinus saw that a light ray passing through calcite was divided
into two rays.
Even when the incident ray was perpendicular to the
face of the calcite crystal, as in Figure 1, one of the rays deviated
This effect was called birefringence.
A transparent material which was isotropic, i.e., a material
with equivalent optical properties throughout, would not have divided
the ray.
Calcite, an anisotropic material, i.e., a material with
optical properties which varied with direction in. the crystal, split
the incident ray.
2.2
The Index of Refraction as a Measure of Birefringence
It is established that light traverses a vacuum with a
uniform velocity of 3 x 10
10
'
cm/sec.
When light travels into
"transparent" matter as air or glass it is retarded to a degree
characteristic of the matter.
Air and other gases hinder light so
little that we usually ignore the effect.
On the other hand, the
retardation of light by air is appreciable under some conditions,
and is, for example, responsible for "mirage" images on deserts.
Matter retards light, and we measure the retardation by the
index of refraction, n, which is always greater than one and equals.
L
=
light ray
Le =
extraordinary ray
Lq =
ordinary ray
Iceland spar
Figure 1.
The double refraction of light by calcite.
'
7
Velocity of the light in a vacuum
Velocity of the light in the material
^
In a birefringent crystal such as anisotropic calcite the velocity
of light depends on the light's direction of travel and the ve
locities, and hence the indices of refraction, of the ordinary and
extraordinary waves differ.
other.
One of the waves is retarded behind the
The difference between the index of refraction of the extra
ordinary ray, n g , and the index of refraction of the ordinary ray,
n Q , is also called birefringence.
Birefringence
2.3
=
n g - nQ
(2)
Huygens' Misconception of Light Waves
Huygens (Halsey 1963) devised a geometrical construction
which predicted the paths of the rays in a doubly refracting .
(birefringent) medium like calcite.
However, he mistakenly believed
that the wave vibration of light was, as it was for sound waves,
longitudinal (Fig. 2), and he could not explain the existence of the
extraordinary and ordinary waves.
One hundred years later Young (Halsey 1963) hypothesized
that light waves were transverse (Fig. 3).
wave it was a simple deduction that
a
From the transverse
birefringent crystal separated
light, represented by the vector E, into two vector components whose
vibrations took place in mutually perpendicular planes
(Fig. 4).
S
=
sound wave:
the arrow represents the
direction of travel of the wave
D
=
vector representing the amplitude of
the sound wave
f Tim®
Figure 2.
The longitudinal vibration of sound waves.
Figure 3.
L
=
light wave:
the arrow represents the
direction of propagation of the wave
E
=
"electric" vector representing the
amplitude of the light wave
The transverse vibration of light waves.
Figure 4.
The two perpendicular components,
and E^, of the electric vector, E.
M
o
As shown.in Figure 4, both components of E--E^ and E — are
perpendicular to the direction of travel of the wave.
With tlie
longitudinal vibration that Huygens assinned, it was not possible
to obtain two perpendicular vector components without one of them
being parallel to the direction of travel of the wave.
Thus
Huygens could not explain the splitting of the incident ray into
the ordinary and extraordinary waves.
2.4
The Nature of Light
Polarized light is necessarily employed in measuring the
birefringence of a glass.
To lead up to discussing the relation
between polarized light and birefringent material in the next section
this elementary background on the characteristics of light has been
included.
Light is electromagnetic radiation, and an electromagnetic
wave like light has perpendicular electric and magnetic fields
represented by the vectors E and M (Fig* 5). .Theoretical and
experimental evidence indicates the electric vector, rather than the
magnetic vector of light, is responsible for all the effects of
polarization and birefringence.
The magnetic vector is therefore
not considered in this discussion.
All light originates from the accelerated motion of electrons
(Ernsberger 1970).
An electron, being electrically charged, is
always surrounded by an electric field.
This field may be thought
of as straight lines, radiating from the electron to infinity in all
Figure 5.
E
=
electric vector
M
=
magnetic vector
L
=
light wave
The electric and magnetic vectors of a light wave.— The
light wave, L, is traveling in a direction perpendicular
to the page.
13
directions.
A two dimensional representation of this is seen in
Figure 6.
In matter the electron oscillates, and, similar to ripples
moving Outward from where a stone dropped into water, there is
undulation of the electric field lines (Fig. 7).
These waves or
.
"kinks" in the electric field lines are light (Ernsberger 1970).
The kinks have a maximum amplitude in directions perpendicular to
the oscillation axis> and this amplitude is represented by the vector
Eq
(Fig. 7).
The direction of E q is (1) perpendicular to the direc
tion of propagation of the light wave, and (2) parallel to the
oscillation axis, YY.
At any intermediate angle, theta, between the
axis of oscillation and direction of propagation the amplitude of
the vector will be E = E^sinO.
E is called the electric vector.
It
should be noted that in Figure 7 there is no wave along the axis of
oscillation, YY.
This means the intensity of light emitted in the
direction of oscillation is zero.
An actual light source consists of many oscillating electrons
which emit light waves with electric vectors oriented in various
directions.
At an instant in time the electric vectors of a beam of
light, L^, coming toward you would appear as in Figure 8.
Because
light is a transverse wave, each electric vector is perpendicular to
the w a v e ’s direction of travel (Fig. 3).
has two components, E^ and E^ (Fig. 4).
In Figure 8, each E vector
All of these E^ and E^
components may be vectorally summed to arrive at single values for
14
Figure 6.
Two dimensional representation of the electric field lines
of an electron, e, after Ernsberger (1970).
15
Y
Y
Figure 7.
Field of an oscillating electron after Ernsberger (1970).-E is the electric vector of a respective light w a v e .
16
II
Figure 8.
Reduction of the electric vectors of a light ray, L, to
one equivalent vector, E^.
17
E
x
and E .
y
The sum values of E
x
and E
y
are the components of a
r
new vector, E^ (Fig. 8).
If the E^ component of E^ were in some way removed, we would
have polarized light.
The E^ component--the electric vector of the
polarized light--is represented by two components E^ and Eq .
2.5
Polarized Light and Birefringent Material
In this study the objective of the experimental procedure was
to measure the effect a birefringent material, stressed isotropic glass,
had on polarized light.
This section is intended to provide a sketch
of polarized light and its interaction with birefringent substances.
When a polarized light wave, L^, strikes a birefringent material
it travels through the material as two wavefronts
The electric vector,
E ^ , (or E^) of the wave consists
dicular components or vectors, E and E .
r
e
o
vectors of wavefronts
E
e
and E
o
and Lq (Fig. 9).
and Lq (Fig. 8).
E
e
and E
o
oftwo
perpen
are theelectric
The linear components
are defined
Ee
=
A esin(wt + ge)
(3)
Eo
=
Aosin(wt + S0)
C4 )
Ae
=
maximum amplitude of
Aq
=
maximum amplitude of Lq wave
w
=
27t (frequency of wave)
t
=
time
ge
=
phase of
g
=
phase of L» wave
o
wave
=
27rf
wave (n, 2tt, etc.)
o
18
Birefringent crystal
/ O
z
Figure 9.
Division of an incident beam of polarized light, L^, into
two wavefronts,
and Lq , upon entering birefringent
material.
19
If the Lg and Lq waves are (1) 0, 180 or 360° out of phase the light
is linearly or plane polarized (Fig. 10),
(2) 90 or 270° out of
phase the light is circularly polarized (Fig. 11),
(3) out of phase
by any amount other than 0, 180, 360, 90 or 270° the light is
elliptically polarized (Fig. 12).
Basically, it is linearly polarized light that is utilized
and analyzed in the experimental procedure of this study; hence, as
an aside, mentioning everyday instances of this "type of light" might
be beneficial.
An interesting one is the navigation of the honeybee
by linearly polarized light.
Evidently, dependent on the position
of the sun, areas of sky are linearly polarized in varying degrees
throughout the day.
The bee navigates through recognition of these
areas as one may navigate by the stars, and the celebrated dance a
scout bee does for his co-workers to point the way to the clover is
based on this recognition. ,Another instance of this sort is the
rainbow.
As you look at a rainbow you view linearly polarized light.
When, as occurs in the experimental procedure of this research,
a beam of linearly polarized light--light with the
and Lq waves
in phase--passes through a birefringent crystal, the chance is small
that the crystal will retard the
or L
■ o
wave exactly 180 or 360
behind the other so the light will leave the crystal linearly polar
ized.
Thus, linearly polarized light entering a birefringent
crystal usually exits as elliptically polarized light (Fig. 13).
20
2 tt \
L
e
wave:
E
e
= A sinfwt + g )
e
&e
E, = E + E
1
e
o
ge = go
Ae ^ Ao
127T
L wave:
o
E
o
= A sin(wt + g )
o
°o
L
In the above
example L and
L are out of
pfiase by 0,
180 and 360°.
and L out
e
o
of phase by
°o
180
360°
tttt
End View
Figure 10.
Linearly or plane polarized light.--The top half of the page
shows the relation between the perpendicular components, L
and Lq , of the polarized light ray,
. The bottom half e
pictures the movement of the electric vector, E^, about the
light ray, L^.
The end view would be seen looking at
straight on, as is the eye.
21
2tt
7 ^
L
L
e
wave:
E
e
= A sin(wt + g )
e
v
E
1
= E
e
+ E
o
S0it/2
8e =
A e ^ Ao
L
o
w ave:
E
o
= A sin(wt + g )
o
o
L
In the above
example Le and
L0 are out of
phase by 90°.
and L out
e
o
of phase by
90°
270
— IT
End View
A
TT
2
Figure 11.
0 27T
Circularly polarized light.--The top
half of the page shows the relation
between the perpendicular components, L and L , of the polar
ized light ray, L^. The bottom half pictures ?he movement of
the electric vector, E,, about the light ray, L^. The end
.
..
11 1
straight on, as is the eye.
view
would
be- seen looRing at
22
wave:
Ee = \ s i n ( w t + ge)
E 1 = Ee + Eo
8e ^ go
A e ^ Ao
2 tt,
wave
-ir
E
o
= A sin(wt + g )
o
&o
L
In the above
example Le and
L are out of
phase by 45°.
0
and L out
e
o
of phase by
Any amount
except 0, 180,
360, 90 and
270°.
TT
-^TT
-IT
2 tt
End View
A
Figure 12.
Elliptically polarized light.--The top
half of the page shows the relation
between the perpendicular components, L and L , of the polar
ized light ray, L^. The bottom half pictures ?he movement of
the electric vector, E^, about the light ray,
. The end
view would be seen looking at L1 straight on, as is the eye.
23
Birefringent crystal
Elliptically
polarized light
Linearly polarized
light
Z
Figure 13.
Linearly polarized light ray, L^, becoming elliptically
polarized by traversing a birefringent crystal.
24
2.6
The Photoelasticity of Glass
Subsequent to Bartholinus' observation of birefringence in
calcite, the next notable discovery towards understanding birefrin
gence was made by Brewster in 1814.
Ideally, "homogeneous glass" is (1) free of composition
gradients and entrapped gas bubbles, and (2) has no residual stress.
Homogeneous glass is optically isotropic and is not birefringent,
and linearly polarized light entering a homogeneous piece of glass
leaves the glass linearly polarized (Fig. 14).
Sir David Brewster (1814) discovered that a piece of homo
geneous glass placed in stress (Fig. 15) became anisotropic and
birefringent.
When an isotropic material like homogeneous glass
becomes anisotropic and birefringent under stress it is termed
photoelastic.
The explanation of why this occurs is called the
theory of photoelasticity.
■
i
Figure 16 presents a physical representation of the photo
elasticity of glass.
The forces P compress the glass in the direc
tion OY and extend it equal amounts in the directions OX and OZ, and
the atoms are pressed closer together in the OY direction and spread
further apart in the directions OX and OZ.. Plane polarized light
entering the glass normal to the direction of thrust, OY, is separated
into two perpendicular wavefronts,
and L^.
Because the glass is
now birefringent, one of the wavefronts is retarded and the light .
exits elliptically polarized (Morey 1954).
25
Y
[omogeneous glass
Linearly polarized
light
Linearly
polarized
light
/
O
z
Figure 14.
Linearly polarized light ray, L^, passing through isotropic
glass.
26
Homogeneous glass
Elliptically
polarized
light
Linearly polarized
light
Z
Figure 15.
Homogeneous glass acquiring the properties of a uniaxial
anisotropic crystal by being subjected to uniaxial
compression, P.
27
Y
yHomogeneous glass
Z!
Linearly polarized
light
Elliptically
polarized
light
jl
r
r
z
Figure 16.
Deformation of a glass block under uniaxial compression, P.
28
In Figure 16 the cube has been distended in the OX and OZ
directions.
The light ray, L^, travels into a cube face which has
b e e n 'compressed in one direction, OY, and distended in the other
direction, OZ.
If the linearly polarized light were to enter the
top of the block, parallel to the OY axis and the force P, it
would exit, as plane polarized light because the cube is distended
equally in the OX and OZ directions; i.e., the glass is "optically
isotropic" in the OX and OZ directions.
A light ray entering the
cube at any other angle would depart elliptically polarized.
2.7
The Theory of Photoelasticity
Brewster's discovery led to the development of the theory
of photoelasticity by Neumann (1841) and Maxwell (1852).
As follows,
the photoelastic theory is the basis of the experimental procedure
and determination of the stress optical coefficient in this
investigation.
If r represents the retardation of one of the waves
or L q
behind the other as the polarized light travels through a birefringent
crystal then,
r
=
n
= index of refraction of the
6
n
n
e
- n
(ne - n0)d
o
(5)
extraordinary
Le
= index of refraction of the
ordinary ray, L
d
= thickness of glass traversed by light
O'
= birefringence
. ■
■
o
29
2
(ne - no ) is proportional to the elastic stress, T (lbs/in ),
(6)
C"e " no)
=
CT
C
=
C
=
a constant depending only upon
the material and the wavelength,
X, of the light
the stress optical coefficient
Combining Equations (5) and (6),
r
CTd
(7)
Equation (7) holds for compression and tension (Savur 1925) and is
assumed valid up to the breaking stress of the glass (Filon 1936)..
Mueller (1935), in explaining why glasses become birefringent
under pressure, outlined two effects that occurred on the atomic
level.
They were (1) the lattice effect:
a decrease in the spacing
of the atoms in the direction of the stress, and (2) the atomic
effect:
a distortion of the atoms themselves (Fig. 17).
birefringence
(2)
positive birefringence
(8)
negative birefringence
(9)
The lattice effect increases the atom density seen by the extraordinary
ray and decreases the atom density seen by the ordinary ray; hence, it
adds to positive birefringence.
The atomic effect increases the atom
density seen by the ordinary ray and contributes to negative
30
o o
o o
o o
o o
0,0
fp
Unstrained
Strained
o o
Lattice effect.
Distances
between atoms decreased.
Positive birefringence.
o o
o o
o
o
Atomic effect.
Spherical
electron clouds are
distorted into ellipsoids.
Positive and negative
birefringence.
o o
° t p 0
Unstrained
Figure 17.
Strained
Mueller concepts of "lattice effect" and "atomic effect"
for glass under compressive force, P, after Ernsberger
(1970).
31
birefringence.
The atomic effect usually outweighs the lattice
effect and glass in compression has negative birefringence.
2.8
The Variation of Birefringence with Composition
Wertheim (1854) was the first to test the laws of photoelas
ticity for a block of glass under simple tension or compression.
He
devised apparatus for producing uniform compression or tension in a
glass block, something Brewster was unable to do, and demonstrated
that the retardation, r, was a function of (Td), where T is the stress
and d the glass thickness traversed by the light.
Wertheim and
following investigators measured birefringence with apparatus similar
to or duplicating that utilized in this thesis (See Chapter 4:
Optical System and Measurement Procedure).
The first determination of the stress optical coefficient,
C, was made by Kerr (1888).
The effect of the chemical composition of glass on bire
fringence was initially investigated by Pockels (1902).
He concluded
that an increase of lead oxide or boric oxide always lowered the
stress optical coefficient.
Filon (1907) and Adams and Williamson (1919) repeated
Pockels' measurements and obtained equivalent results.
Adams and
Williamson compared their data with Pockels' as in Figure 18.
Vedam (1950) measured the photoelastic properties of 18
optical glasses, but gave only approximate glass compositions.
An
4
A Adams & Williamson Glasses
O Pockels Glasses
-4 * ' — 1—
30
40
1.1
50
W e ig h t %
Figure 18.
60
Lead
. ..I— .70
80
O x id e
Comparison of Adams and Williamson's and Pockels' data of birefringence vs.
composition for flint glasses after Adams and Williamson (1919).
(X
K)
33
inclusive study of photoelastic properties was made for 154 optical
glasses by Schaefer and Nassenstein (1953).
The glasses were .
produced by Schott and Company and Schaefer and. Nassenstein did not
include glass composition data in their paper.
Finally, the stress optical coefficients of 27 optical
glasses made at the National Bureau of Standards (NBS) were deter
mined by Waxier and Napolitano (1957).
data were listed.
Definitive glass composition
The NBS data was comparable to measurements made
50 years before by Filon (1907) and PoekeIs (1902)(Fig. 19).
2.9
Application of Data on the Variation of
the Stress Optical Coefficient with Composition
While it has been demonstrated that the stress optical
coefficient varies with composition, birefringence has not been
shown to vary linearly in phase fields, and the literature does
not reveal a procedure which can accurately predict the stress
optical coefficient of a given glass.
Babcock (1968) segregated published data on refractive index
into primary phase fields for various binary and ternary glass
systems.
He then subjected the data to least-squares analysis on a
IBM 1620 computer.
The computer presented an expression, an equation
which allowed the refractive index to be calculated, of the form:
Glass Property
=
A SiOg + B Na^O + C CaO + ...
A, B, C ...
=
numerical constants, calculated by the
computer for a given phase field,
characteristic of the respective oxides
(10)
Brewsters
in
O NBS Glasses
□ Pockels Glasses
A Filon Glasses
O
20
40
W e ig h t %
Figure 19.
60
80
100
Lead O x id e
Variation of stress optical coefficient, C, with glass composition after Waxier
and Napolitano (1957).
Cn
4^
35
Si02 , N a 20, CaO
amounts of these oxides, expressed
in mole fractions
The calculated indices of refraction showed the refractive index
varying linearly within phase fields of the glass system.
Figure 20
shows the linear variation of refractive index within phase fields
of the Na20-Ca0-Si02 glass system.
The standard error.
(11)
,
ANp
=
difference between (1) published data submitted
to the computer and (2) the data calculated by
Babcock using Equation (10)
n
=
number of data points
was less than 0.0016 in all cases.
Babcock (1968, 1969) applied this technique with.equal success
to the glass properties of density, viscosity and thermal.expansion.
Figure 21 pictures the linear variation of Knoop hardness within four
phase fields of the Na20-Ca0-Si02 glass system.
Equations employed
to calculate data shown in the N2S and N2C3S phase fields of Figures
20 and 21 are given in Figure 22.
The advantage of Babcock's formulation is as follows.
Say a
glass of the following specifications is required in the Na20-Ca0~Si02
system
Refractive index
=
1.623
Density
82.4 lb/ft
Viscosity
100 poise
3
36
N3C6S
N2C3S
CaO
(mole
fraction)
BCS
N*
N2S
SiOa (mole fra c tio n )
Figure
Linear variation of refractive index in phase fields of the
Na^O-CaO-SiO^ glass system after Babcock (1968).
37
Beta
CaO • S iO )
\
TRIDYMITE
N a g O 3CaO 6 SiO
(mole
frac tio n )
u»
CaO
N a 20*2Ca0-3Si02
S i 0 2 (m ole fra c tio n )
Figure 21.
Linear variation of Knoop hardness in phase fields of the
Na20-Ca0-Si02 glass system after Georoff (1972).
38
A.
B.
In the N2S phase field:
1.
Refractive index = 1.4739 Si02 + 1.7816. CaO + 1.5658'Na20
2.
Knoop hardness
In
= 102.69 Si02 + 2633.0 CaO + 839.10 Na20
the N3C6S phase field:
1
1.
Refractive index = 1.4715 Si02 +
1.7638 CaO
+ 1.5740 Na20
j
2.
Knoop hardness
874.02 CaO
- 49.460 Na20
j
= 525.23 Si02 +
.
Figure 22.
Equations used to calculate data plotted in the N2S
and N3C6S phase fields in Figures 20 and 21 after
Babcock (1968) and Georoff (1972).
39
Knoop hardness
=
490
These glass properties along with the A, B, and C constants for each
(1) primary phase field and (2) glass property, are submitted to the
computer.
The computer attempts to arrive at a glass composition of
X mole fraction of Na20, Y mole fraction of CaO and Z mole fraction
of SiOg, that will produce a glass with the above properties.
In
other words, the computer determines the composition per glass
specifications.
This procedure has advantage over melting, polishing
and testing a series of glasses to obtain a sample to meet required
properties.
In addition, if a composition is known, the computer
reverses the process, and, minus the usual laboratory investigation,
predicts the physical properties of the glass.
2.10
In Review
On the preceding pages we have seen
1.
The discovery of birefringence
2.
The discovery of the photoelasticity of glass
3.
The development of the theory of photoelasticity
4.
The variation of the stress optical coefficient
composition
5.
The prediction of physical properties of glass by Babcock1s
formulation
with
In this paper the question at hand is whether the stress optical
coefficient also has a linear relation within phase fields and
can be added to the list of predictable glass properties.
CHAPTER 3
PREPARATION OF GLASS SAMPLES USED TO MEASURE BIREFRINGENCE
The majority of glasses h a v e .a stress optical coefficient
of two to three brewsters, where a brewster equals a relative
retardation (of the ordinary ray behind the extraordinary ray) of
one angstrom unit per millimeter per bar.
This small value, the
retardation between the extraordinary and ordinary rays, is the
quantity measured in this study.
Because excessive residual strain
or slight deviations in a glass' composition can mask a glass' true
stress optical coefficient, it was necessary to produce glasses
with negligible residual strain and with a high degree of
compositional homogeneity.
The factors affecting the compositional homogeneity Of the
glasses used were
(Babcock 1959),
1.
Purity of the raw materials, and
2.
their grain sizes and their distribution
3.
Blending technique
4.
Batch size
5.
Extent of crushing
6.
Extent of stirring
7.
Time and temperature of melting
40
41
An additional factor, the annealing process, determined the amount
of residual strain remaining in the glass at room temperature.
Figure 23 demonstrates where the above factors came into play in
the sample preparation procedure.
A discussion of the preparation
of glass samples and the role of the above factors therein follows.
3.1
Weighing of Glass Constituents
A 1200-2000 gram batch was weighed on the Torsion balance
(for further description of the equipment used in preparing glass
samples, see Appendix C ) .
each constituent used.
See Tables 1-4 for the mole fraction of
Reagent grade materials were employed
(See Appendix B for chemical analysis of these materials).
See
the thesis by Georoff (1972) for photographs of the equipment
employed in the preparation of glass specimens.
3.2
Mixing of Glass Constituents
The v
b atch was put into a plastic bag and mixed in a U.S.
Stoneware porcelain ball mill.
The reagents were in powder form
of 50-100 mesh (300-150 microns) and further pulverization was
not required.
3.3
Melting the Batch
After blending the mixture was poured into a platinumrhodium crucible and placed in the Pereny furnace.
This was done
in increments so gases generated on heating would not cause the
powder, to spill out of the crucible onto the furnace floor.
Each
.
Step
Sample Preparation Procedure
42
Key Factors in Glass Homogeneity
Glass Constituents Weighed
Purity of raw materials
Glass Constituents Mixed
Grain sizes and distribution
Blending technique
Batch Melted
Batch size
Glass Quenched, Washed and
Dried
Extent of crushing
Extent of stirring.
Time and temperature of
melting
Batch Remelted; Stirred
3.6
,
Glass Poured into Mold
8.
Glass Annealed
Annealing process
Composition Uniformity and
Residual Strain of Glass
Checked
Overall Composition
Checked
1 x 1 x 3 cm Samples Cut
from Glass and Ground and
Polished
r- — r
-----
1
j 3 . 1 1 j Stress Optical Coefficient
i
I of Glass Measured
i
Figure 23.
Preparation of a glass sample and important factors
affecting glass homogeneity.
43
Table 1.
Chemical Composition and Critical Temperatures for Glasses
Investigated in the D (Unknown) Phase Field of the SodaTitania-Silica Ternary System
D2
D3
04
0.650
0.600
0.600
0.670
0.150
0.150
0.200
0.120
0.200
0.250
0.200
0.210
1300
1340
1400
1350
Annealing temp (°C) corresponding temp to
viscosity = 1 0 ^ poise
610
565
600
580
Liquidus temp (°C)
845
883
928
900
. Glass
D1
.
Composition (mole fraction)
Si02
Ti02
Na20
Melting temp (0C) corresponding temp to
viscosity = 10^ poise
44
Table 2.
Chemical Composition and Critical Temperatures for Glasses
Investigated in the E (NagO-TiO^'SiOg) Phase Field of the
Soda-Titania-Silica Ternary System
Glass
El
E2
E3
E4
0.500
0.450
0.400
0.550
Ti02
0.200
0.250
0.300
0.200
Na20
0.300
0.300
0.300
0.250
1280
1250
1300
1250
Annealing temp (°C) corresponding temp to
viscosity = 10^^ poise
550
520
535
550
Liquidus temp (°C)
912
922
920
890
Composition (mole fraction)
Si02
Melting temp (°C) corresponding temp tp
viscosity = 10^ poise
:
45
Table 3..
Chemical Composition of the Five Commercial Laboratory
Melted Glasses in the Nepheline and Corundum Phase Fields
of the Soda-Alumina-Silica Ternary System
Glass
A1
A2
A3
A4
.
AS
0.620
0.620
0.620
0.620
0.620
0.100
0.150
0.190
0.205
0.220
0.280
0.230
0.190
0.175
0.160
Composition
(mole fraction)
Si02
A12°3
Nao0
46
Table 4.
Chemical Composition and Critical Temperatures for Glasses
Investigated in the Nepheline Phase Field of the SodaAlumina-Silica Ternary System
Glass
A6
A7
Composition (mole fraction)
s i o 2
0.650
. 0.590
A 1 2 ° 3
0.125
0.125
N a20
0.225
0.285
1475
1500
600
625
1000
1050
Melting temp (°C) corresponding temp to
viscosity -= 10^ poise
Annealing temp .(°C) corresponding temp to
viscosity = lO-*-^ poise
Liquidus temp (°C)
47
glass was melted at a temperature equivalent to log 2 viscosity
(Tables 1-4).
Because glasses poured from 600 gram batches had
composition gradients visible with the polariscope, larger batches
of 1200 to 2000 grams were utilized.
The larger batches minimized
surface gradients resulting from (1) the interaction of molten glass
with the sides of the platinum-rhodium crucible and (2) volatiliza
tion of NagO into the furnace atmosphere.
3.4
Quenching, Washing and Drying the Glass
Two or three hours following the final batch increment
added to the Pereny furnace, the glass was poured between water
cooled Fafnir aluminum rollers to form a thin sheet of glass which,
upon passing through the rollers, fell into a pan of distilled
water.
This quenching technique caused the glass to fracture into
tiny fragments and effectively mix itself.
The glass was quenched
in distilled water as tap water would have contaminated the glass
with soluble salts which cause glass particles to adhere, hindering
mixing.
The distilled water was decanted and the glass mixture
washed with alcohol and dried on a Corning laboratory hot plate.
The highest degree of homogeneity from this m e 11ing-quenching
technique was achieved after two or three repetitions.
Furthbr
melting and quenching did not improve the glass’ homogeneity.
3.5
Remelting and Stirring the Glass
Subsequent to the final quench the batch was remelted,
left in the furnace until free of bubbles, and, shortly before it
was poured, stirred in an attempt to blend the surface layer that
formed from.Na^O volatilization.
In comparison with quenching,
stirring was relatively ineffective in mixing the glass.
The
object was to leave the glass in the furnace for a time short enough
to minimize surface volatilization and long enough to minimize seeds
(bubbles).
3.6
Pouring the Glass
Thirty to forty minutes after the final stirring, the glass
was poured into a stainless steel mold which gave a cylindrical
sample three inches in diameter and three-quarters of an inch
thick.
The mold was tapered from top to bottom to facilitate
removing the glass disk.
3.7
Annealing the Glass Disk
A minute or two after being poured, the glass would solidify
such that it would not sag when removed from the m old.
At this time
it was transferred to the Lindberg annealing furnace on an asbestos
board and cooled at 2.5°C per hour from its annealing temperature
(Tables 1^4) to 345°C.
At 345°C the furnace was shut down and
allowed to cool to room temperature at about 20°C per hour.
total annealing time per glass was. approximately 110 h o urs.
The
49
When a glass is poured into a mold, the peripheral surface
solidifies first, the center last.
The outer crust is then in
compression while the central portion, which tried to contract on
cooling.but was held by the solid crust, is under tension.
If the
glass is allowed to remain at and cool to room temperature after it
has solidified, the internal strains will often cause it to become
riddled with cracks and, at times, to explode into tiny fragments.
This is why a glass is put into an annealing furnace just after it
has solidified.
The furnace slowly decreases the temperature and
allows atoms to rearrange to a more stable configuration.
A
completely strain free glass is attained only by annealing for
infinite time.
At less than an infinite annealing time, residual
strain always remains in the glass.
For the glasses examined in
this paper, a cooling rate of 2.5°C per hour left residual strain
only slightly visible when the glass specimens were examined with
the polariscope.
5.8
Residual Strain and Compositional Uniformity
Residual strain and composition gradients reflect a glass’
(in)homogeneity.
As the measurement of the stress optical coefficient
was extremely sensitive to nonuniform composition and strain, it was
critical that the glasses be tested for these properties.
A Bausch and Lomb vertical polariscope was employed in
analyzing residual strain and compositional variation in the glass
50
disk.
Those portions of the disk with the highest degree of
homogeneity were cut out and polished as samples.
As a final
check prior to being measured for the stress optical coefficient,
the sample was viewed in the polariscope.
5.9
Overall Chemical Composition of the Glass
In 1893 F. Becke discovered that an optically transparent
grain immersed in oil displayed a bright halo concentric with the
grain's border when a microscope objective was focused slightly above
the position of sharpest focus.
This halo moved in a direction
toward the medium (oil or crystal) having the higher refractive index
as the microscope was adjusted upward from the correct focus.
With
these concepts in mind, the refractive index of the crystal could be
determined by immersing it in oils of various refractive indices.
When the refractive index of oil and crystal were equal the halo
disappeared.
The halo, after its German discoverer, came to be known
as the Becke line and the procedure the Becke line method or method
of central illumination.
The Becke line method of measuring the index of refraction
of a material is rapid and economical, and has long been employed
by geologists and chemists in classifying transparent crystals.
accuracy of ± 0.001 is easily achieved with this procedure.
An
Since
the refractive index of glass varies directly with composition,
measuring the refractive index by the Becke line method was chosen
to verify the composition of glasses, melted for this paper.
■ 51
3.9.1
Procedure Followed in Measuring
the Refractive Index of a Glass
About one cubic centimeter of glass was ground to a fine
powder which was sprinkled along a microscope slide.
It is
worthwhile to note that glass, because it is not. malleable like
metals, does not have strain introduced during grinding.
The
slide was inserted on the stage of a Leitz polarizing microscope
and a drop of oil placed on the powder.
If the index of refraction
of the oil was not equal to that of the glass powder, the Becke line
appeared, and, when the microscope was adjusted upward, moved towards
the medium (oil or glass) with the higher index of refraction.
Through trial and error an oil with an index of refraction equal
to the glass * was obtained (Bloss 1961).
3.9.2
Evaluation of Measured Refractive Indices
Making use of a filter whose dominant wavelength was close
to the sodium D line, the refractive indices of the (1) eight sodatitania-silica,
(2) five commercially melted soda-alumina-silica
and (3) two soda-alumina-silica glasses were measured.
In Table 5
the data measured for the soda-titania-silica glasses is compared
to values measured by Hamilton and Cleek (1958) and to values calcu
lated by Babcock (1969).
The indices obtained for the soda-alumina-
silica glasses are listed with values calculated by Babcock (1968) in
Table 6.
All measured data was within ± 0 . 0 0 1 of the values of Ham
ilton and Cleek (1958) and Babcock (1968; Babcock and Georoff 1973).
52
Table 5.
Index of Refraction Values for Glasses in the D and E
Phase Fields of the Soda-Titania-Silica Ternary System
Fully Annealed Glass Samples
Glass
Measured Values
NBS Values*
Calculated Values**
D1
1.602
1.6019
1.6025
D2
1.601-1.602
1.6016
1.6018
D3
1.642-1.643
1.6424
1.6421 :
D4
1.657-1.658
El
1.636
1.6362
1.6360
E2
1.673-1.674
1.6739
1.6733
E3
1.710-1.711
1.7109
1.7105
E4
1.640-1.641
1.6402
1.6407
1.5786
^National Bureau of Standards (NBS) values from Hamilton and Cleek
(1958)
**After Babcock (1968)
53
Table 6.
Index of Refraction Values for Commercial Laboratory
Melted Glasses in the Nepheline and Corundum Phase Fields
of the Soda-Alumina-Silica Ternary System
'f
Glass
.
Measured Values on
Annealed Glasses
Calculated Values*
1.5052
A1
1.506
A2
1.503-1.504
A3
1.501-1.502
1.5020-1.5021
A4
1.504-1.505
1.5043
A5
1.507
1.5066
A6
1.501-1.502
1.5013
.A7
1.507-1,508
1.5074
*After Babcock (1968)
. 1.5035
3.10
At this
Cutting and Finishing of the Glass Sample
point the glass disk was annealed, relatively
homogeneous and of known composition.
The disk was submitted
to the Optical Sciences Center Optics Shop and a sample approximately
1 x 1 x 3
centimeters prepared.
Sample preparation, while
the specimen is
Figure 24 lists criteria used in
the process used in polishing and grinding
described in Figure 25.
The specimens were made
three times as long as they were wide to negate end effects:
(Frocht 1948).
The glass was polished on a standard glass polishing spindle
and head.
RPM.
The spindle speed was 10 RPM, arid the head speed 25-30
The head weight of 10 pounds was reduced to two pounds, when
polishing with milled barnesite.
3.11
The Finished Piece of Glass
The succeeding chapter describes the measurement of the
stress optical coefficient of the completed glass sample.
55
1 cm
1 cm
Q
Sample had two square end
faces and four rectangular
side faces
Qzj
End faces were parallel to
+_ 0.01 inch
fsj
Opposite side faces were
parallel to +_ 0.001 inch
All six sides of sample
were polished
3 cm
Q5J
Figure 24.
The edges were slightly
beveled to facilitate
polishing
Dimensions of the finished sample and criteria used
in its preparation.
Step
Procedure
©
Sample of about 1.00 x 1.00 x 3,00
cm cut from glass, disk
©
Sample generated to about 0.995 x
0.995 x 3 cm by diamond cup wheel
(142 micron grit), with water and
water soluable oil as lubricant
©
Sample polished with ‘30 micron
aluminum oxide slurry
©
Sample polished with 3 micron
aluminum oxide slurry
©
Time(hr)
0.50
.
r
:_____ ......... ...... .
Sample polished with one micron
milled barnesite (cerium oxide
0.75
milled 200-300 hours) and
distilled water
I
(Approximate Time
("stress optical coefficient of
the sample determined
L
Figure 25.
3.00
— 1|
j
Procedure for preparing finished glass samples
CHAPTER 4
OPTICAL SYSTEM AND MEASUREMENT PROCEDURE
The optical arrangement described below was adapted from
that employed by Waxier and Napolitano (1957).
The quantity measured
with this optical system was the retardation, in degrees, of the
ordinary ray behind the extraordinary ray (Fig. 1).
As discussed in
the next chapter, the stress optical coefficient of a glass sample
was calculated from this value.
4.1
The Optical System
A schematic and photographs of the optical bench arrangement
are pictured in Figures 26 and 27.
The objective of the optical
system was to (1) impinge a beam of linearly polarized light on the
sample,
(2) convert the elliptically polarized light leaving the
sample (Fig. 15) to linearly polarized light and,
(3) since the
stressed glass and quarter wave plate in combination have the same
effect as an optically active material that rotates the linearly
polarized light, to measure the rotation of the plane polarized
light by adjusting the analyzer to extinction.
The difference, y
degrees, between the position of the analyzer (1) before it was
adjusted and (2) after it was rotated to extinction, represented the
retardation of the ordinary ray with respect to the extraordinary ray.
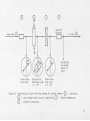
Figure 26.
Optical bench arrangement.
©
©
©
°0
©
0
©
©
©
©
\
U
)
,
11
®
©
@
O
p(>
p
Schematic of apparatus.
H J
=
light source;
=
lens; ( ? )
compression; Q t J
(?)
=
lens;
(To)
=
=
lens; ( T )
polarizer; ( ? )
=
=
=
filter; ^ 3 )
photomultiplier;
pin hole;
glass sample under uniaxial
Babinet compensator; ^ 8 ^
=
=
=
=
polarizer (analyzer);
oscilloscope.
Optical bench arrangement.
above schematic.
The numbers correspond to those in the
in
00
Figure 27.
Continued optical bench arrangement.--The numbers correspond to those in the
schematic in Figure 26.
in
ID
Figure 28 pictures the types of polarized light formed as the light
makes its way through key parts of the optical system.
The apparatus
and the variation of the light in its transit of the optical system
are further discussed below.
4.1.1
Light Source
The original light source was a xenon arc lamp.
Its
beam proved unsteady, and a tungsten strip lamp was substituted
in its place (for equipment details see Appendix D ) .
4.1.2
Monochrometer or First Lens
The monochrometer in Figure 26 was removed because the light
it produced was polarized.
The focusing system of the monochrometer
was then allowed for by the addition of a lens (number ( T ) in the
schematic in Figure 26) with a radius of curvature equal to 70 milli
meters.
This lens, as part of the system which collimated the light
before it passed through the sample, focused an image of the tungsten
strip on the pinhole.
The interference filter (number (jP) in the
schematic in Figure 26), which was originally in the optical system
to eliminate monochrometer light which did not have a wavelength "of
5461 angstroms, gave, by itself, a sufficiently monochromatic light
beam.
4.1.3
Interference Filter
The filter eliminated all wavelengths, X, contained in the
white light produced by the tungsten strip bulb except for a
yellow-green light with a wavelength equal to 5461 angstroms.
©
From lens.
©
p
©
®
Analyzer
Adjusted
4
To lens.
EXTINCTION:
negligible
amount of
light
Plane Polarized Light
Figure 28
Elliptically
Polarized Light
6 t 45°
Plane Polar
ized Light
tf) ^
45°
Polarization of light traveling through the optical system.—
f8j
=
glass sample under uniaxial compression; ^ 7 ^
=
polarizer (analyzer).
=
=
polarizer;
Babinet compensator;
ON
62
4.1.4
Pinhole
The pinhole was employed in collimating the monochromatic
light emerging from the filter.
Light passing through the pinhole
diverged at a constant rate, and a lens placed beyond the pinhole at
a distance equal to the lens' focal length produced a collimated
light beam.
4.1.5
The diameter of the pinhole was two millimeters.
Second Lens
This lens was used in conjunction with the pinhole to. obtain
a collimated beam of light.
millimeters.
The lens' radius of curvature was 94
In selecting lenses for the optical system (1) the length
of the optical bench and (2) the fact that shorter focal length lenses
have more light gathering capability were taken into account.
4.1.6
First Polarizer
A Glan-Thompson polarizer linearly polarized the monochromatic
light exiting the collimating lens.
Figure 29 details how light was
linearly polarized by the polarizer.
The optic axis of the glass was on the line along which the
compressive forces, P, were applied, or, it was essentially vertical.
The polarizer was set at 45° to the optic axis of the glass specimen.
With the polarizer oriented in this fashion, linearly polarized light
entered the stressed sample at 45° to the optic axis and split into
two waves (the extraordinary and ordinary) of equal amplitude (Fig. 15).
Wave vibration
parallel to page
Wave vibration
parallel to page
Calcite
prisms "
Lq = ordinary wave
Le = extraordinary wave
The ordinary light wave is reflected and absorbed
by a blackened side of the polarizer while the
extraordinary wave passes unaltered.
Figure 29.
Gian-Thompson polarizer
64
4.1.7
Glass Specimen Under Uniaxial Stress
The method of applying a uniform compressive stress to the
sample is described in Appendix D.
As explained in Chapter 2, the
linearly polarized light entering the stressed glass exited as
elliptically polarized light.
A piece of black cardboard with a
six millimeter diameter hole was propped against this apparatus and
adjusted back and forth so the light beam passed through the center
of the specimen.
4.1.8
Soleil-Babinet Compensator
Operation of this instrument is explained in Figure 30.
Elliptically polarized light may be considered as composed of two
linear components (1) parallel to the major and minor axes of the
ellipse (Fig. 12), and (2) with a quarter wave phase difference
between them.
Hence, if a Babinet compensator is set as a quarter
wave plate, and the horizontal axis of the compensator placed
parallel to one of the axes of the elliptically polarized light,
elliptically polarized light is, on traveling through the compensator,
converted to linearly polarized light.
.
In this study the compensator was adjusted to act as a quarter
wave plate for light with a wavelength of 5461 angstroms
(Fig. 31).
The compensator axis was then placed parallel to an axis of the
elliptically polarized light by (1) putting the maximum load on the
tare platform and (2) rotating the compensator alternately with the
analyzer until the extinction position was identified.
65
Adjustable
Quartz Wedges
Quartz Plate
Elliptically
Polarized
Lightx
Linearly
Polarized
Lights
OA = optic axis
ordinary ray
Lo =
L = extraordinary
ray
Horizontal Axis of
Compensator
The extraordinary wave, because it is parallel to the optic axes
of the two quartz wedges, is not affected as it passes through the
wedges.
However, it is retarded a fixed amount by the quartz
plate.
The ordinary wave, since it is not parallel to the
the wedges, is retarded in traversing the wedges.
retarded while passing through the quartz plate.
optic axes
It is not
of
To put the extraordinary and ordinary waves in phase, the quartz wedges
were adjusted so the ordinary wave was retarded back into phase with
the extraordinary wave.
Figure 30.
The Soliel-Babinet Compensator.
66
I-
Light in:
X = 5461 A
u
o
<D
4->
cti
to
<D
Ph
E
o
u
N
•H
r—
(
©
o
Oh
Light out = 0
(Extinctioi
Linearly Polarized
©
2 ) Circularly
Polarized
Mirror
LinearlyPolarized
( T ) Circularly
Polarized
If, for the wavelength of light being used, a Babinet compensator is
correctly set as a quarter wave plate, the situation pictured above
occurs:
(l)
©
©
©
Light passing through a polarizer is linearly polarized.
Linearly polarized light is circularly polarized by the
quarter wave plate (Babinet compensator).
The electric vector of the circularly polarized light
rotates in the opposite direction after the light is
reflected by the mirror.
The compensator transforms the circularly polarized
light into linearly polarized light.
The orientation of the linearly polarized light from the
compensator is perpendicular to the orientation of the
polarizer, and the light is extincted.
To adjust the compensator so it acted as a quarter wave plate,
the polarizer, compensator and mirror were arranged_as shown above
and the compensator adjusted until the light ( at (V)) was extincted
The Soliel-Babinet compensator had a scale of -20 to 0 to 20 (forty
divisions), with a circular vernier readable to 0.01 of a division.
It was set at 1.48 for the light (X = 5461 angstroms) employed in
this investigation.
Figure 31.
Setting a Babinet compensator as a quarter wave plate.
67
4.1.9
Second Polarizer or Analyzer
The polarizing axis of the analyzer was "crossed" (placed
perpendicular to) with the axis of the first polarizer.
The
analyzer was mounted in a graduated circle rotator which had a
360° scale and a vernier readable to 0.05°.
4.1.10
Third Lens
.
■
This lens focused light from the analyzer onto the. face of
the photomultiplier tube.
The radius of curvature of the lens was
94 millimeters.
4.1.11
Photomultiplier and Oscilloscope
A voltage supply provided input for the photomultiplier
while the output was exhibited as a straight line on the oscilloscope
screen.
Up or down movement of the straight line with the adjustment
of the polarizer or the Babinet compensator defined points of minimum
light intensity.
Section 4.2 describes how these minimum light ,
intensities established the optic axis of the sample and determined
the retardation of the ordinary ray behind the extraordinary ray.
4.2
Experimental Procedure
As was the glass block in Figure 15, the glass specimens in
this study were placed under a uniform compressive stress and acted as
birefringent crystals, thus causing the ordinary wave to lag behind
the extraordinary wave.
This retardation, in degrees, was the quan
tity measured by the procedure discussed below.
68
4.2.1
Alignment
The interference filter used (number (T) in Figure 26) had
a mirror surface on one side, and, as a base point from which to begin
alignment,
(1) the filter holder, pin and optical bench slide were
removed from the optical bench, and (2) the mirror face put parallel
to the front edge of the slide base with a bubble level.
In other
words, the mirror face was oriented perpendicular to the lengthwise
horizontal axis of the optical bench.
'
The mirror, pin and slide combination was then remounted on
the optical bench and adjusted in tandem with the light housing (which
did not mount on the optical bench) so:
1.
When the filter mirror was at the end of the optical bench
farthest from the housing, the beam of light emitted by
the housing was centered on the mirror.
2.
When the filter mirror was at the end of the optical
bench closest to the lamp housing, the mirror's reflection
of the tungsten strip image was centered on the iris of
the housing.
The circular opening of the iris was adjust
able from two to forty millimeters.
With the filter mirror
one inch from the housing and the iris stopped down to two
millimeters, an image of the tungsten strip about two
millimeters wide and ten millimeters in length was visible
on the black iris face.
3.
If both (a) the reflected filament image was centered on the
iris, and (b) the light beam was centered on the mirror when
69
the interference filter was at the far end of the bench,
the light source was assumed on line with the optical
bench.
Other parts of the optical train were then added
to the bench and adjusted to be perpendicular to the light
path by noting that the reflected beam of light coincided
with the incident beam.
During the preliminary attempt at aligning the optical system, the
filament of the tungsten light was not directly centered on the iris,
but was slightly off center towards the upper part of the iris.
This
caused the light to "sink" as it progressed down the optical bench.
So the light beam would travel parallel to the optical bench,.the
light housing was disassembled and the filament centered on the iris.
4.2.2
Orientation of the Polarizers
In order to position the polarizers at 45° relative to the
optic axis, the glass sample was loaded to the maximum weight of
35.5 pounds, and both the polarizer and analyzer rotated until, as
noted on the oscilloscope, a point of minimum intensity was reached.
For each sample four such positions, each about ninety degrees from
-
the other, could be found.
Only two of these, within 90
of each other,
were required to position the polarizers.
The two points of minimum intensity represented the positions,
of the vertical and horizontal axes of the extraordinary and ordinary
rays respectively.
After the points were identified, the specimen was
removed and the analyzer set at a position halfway between the two
70
points.
Finally, the first polarizer was rotated to an extinction
position, and, as was originally desired, the polarizers were
crossed and at 45° relative to the optic axis.
During the above procedure all readings were taken from
the graduated circle rotator in which the analyzer was mounted.
The
extinction positions noted on the graduated circle scale were repro
ducible to within ± 0.2 degrees.
From Goranson and Adams'
(1933)
discussion on possible errors introduced by missettings, this
appeared to be acceptable.
The Babinet compensator was removed while the optic axis
was located and the polarizers crossed with each other.
After the
compensator and glass specimen were reinserted, the apparatus was
ready to measure the stress optical coefficient of the glass sample.
4.2.3
Measurement of the Retardation of
the Ordinary Behind the Extraordinary Ray
When the glass specimen was replaced, the maximum weight of
35.5 pounds was put on the loading platform, and the
analyzer and
Babinet compensator (set as a quarter wave plate)alternately
rotated
until the position of minimum intensity was determined, or, until the
horizontal line on the oscilloscope screen was at its lowest point.
This procedure was repeated six times.
The six measurements were
averaged to obtain a value, y degrees, for the extinction point.
An increment of 5.5 pounds was then removed and the analyzer
rotated to the minimum light intensity.
made at this position.
Six measurements were also
Extinction positions were defined for four
71
more weight reductions of 5.5 pounds, giving a total of six extinction
positions of y degrees.
Table 8 shows how the differences. Ay,
between these six points were averaged to give Ay, the retardation
in degrees of the ordinary behind the extraordinary ray.
Measurements were performed in a dark room at a temperature
of about 20oC, and, after removing a 5.5 pound increment, five minutes
were allowed to lapse before, the new extinction position was determined.
During preliminary measurements, 5.5 pound increments were
added to the platform and the extinction position noted.
However, at
lower weights residual strain and small composition differences masked
the position of the axes of the elliptically polarized light emerging
from the sample, and with each addition of 5.5 pounds the compensator
had to be readjusted with the analyzer to get a true minimum, intensity.
Data (y values) taken in this manner plotted more as a curved line
(Fig. 32).
This effect became negligible at higher weights; hence,
the compensator was rotated into position with the maximum load applied.
Once the Babinet compensator was positioned at the maximum load, re
sults were independent of whether measurements were made by (1) be
ginning with minimum weight on the platform and adding 5.5 pound
increments or by (2) starting at the maximum load and removing 5.5
pound increments.
1.
In summary:
The Babinet compensator was set as a quarter wave plate as
shown in Figure 31.
Since the same wavelength of light was
used throughout the study, the compensator remained at this
setting for the entire investigation.
72
290
288
Babinet compensator adjusted
at each extinction point----
286
k
284
282
280
278
Babinet compensator positioned at the maximum
load and kept in this position for the
remainder of the measurements
276
I
13.5
24.5
19.0
W eight,
Figure 32.
1
i
in
29.0
1
34.5
pounds
Affect of the positioning of the Babinet compensator on
extinction points (y values) obtained.--The above
measurements were made on glass A2.
2.
Whenever a new sample was placed in the loading apparatus,
(a) the optic axis had to be determined,
(b) the polarizers
crossed, and (c) the compensator repositioned at maximum
load.
3.
.
For an individual sample:
once the compensator was rotated
into place at maximum load, it stayed at that position for
the remainder of the measurements.
If the glass specimens had had a residual tensile stress, this
would have had to be overcome by an equal compressive force before the
birefringence measured would have been due only to compressive stress.
In accordance with the possibility of residual tensile strain, four 2
pound lead weights were laid across the loading platform before the
five 5.5 pound weights were added.
The credibility of the optical system was tested using a
National Bureau of Standards glass with a stress optical coefficient
measured by Waxier and Napolitano (1957).
The. value obtained was within
1.51% of the stress optical coefficient arrived at by Waxier (Table 7).
As an example. Table 8 illustrates all data taken during the
measurement of a glass sample.
Tables 9-11 list the stress optical
data for the glasses investigated.
The succeeding chapter outlines
how, from the Ay values listed in these tables, stress optical
coefficients were calculated and analyzed.
74
Table 7.
Calibration of the Optical System through Measurement
of the Stress Optical Coefficient of National Bureau
of Standards (NBS) Sample BF 588
Data Relating to Measurement
of the Stress Optical Coef
ficient of NBS Sample BF 588
Measured stress optical
coefficients, C, in
brewsters
Values Obtained from
Optical System Employed
in this Investigation
' 2.3134, 2.3120,
2.3206, 2.3119,
2.3149
NBS
Values*
---
Average stress optical
coefficient, C, in
brewsters
2.3145
2.35
Standard deviation among
measured stress optical
coefficients, C
0.0034
0.026
Wavelength of light, in
angstroms, employed in
measuring stress optical
coefficients, C
5461
5893
Compressive stress in
crements, in lbs/cm^,
added to glass specimen
during measurement of
stress optical coef
ficients, C
5.5
5.5
*After Waxier and Napolitano (1957)
Table 8.
Data Recorded in Measuring the Stress Optical Coefficient of Soda^Alumina-Silica Glass A3
Weight on Load
ing Platform,
in Pounds*
35.5
Retardation
Values, y,
in Degrees
287.150,287.050
287.150,287.000
287.125,287.095
Average Retar
dation Values,
y, in Degrees
Ay Values,
in Degrees
Average Ay
Value, Ay,
in Degrees
Calculated Stress
Optical Coefficient,
C, in Brewsters.
=
287.095
2.524
30.0
284.625,284.650
284.525.284.525
284.575.284.525
284.571
2.488
24.5
282.125,282.075
282.150,282.025
282.075,282.050
. 282.083
2.495
19.0
279.575.279.600
279.575.279.600
279.575.279.600
2.5114
2.9535
279.588
2.546
13.5
277.050.277.025
277.075.277.025
277.075,277.000
277.042
2.504
;8,o
274.600,274.475
274.450,274.625
274.625,274.450
274.538
-
*The positions of the vertical and horizontal "optic" axes were 316.950 and 226.925° respectively.
The Babinet compensator was set midway between these values at 271.940°.
Table 9.
Glass
A1
A2
A3
A4
Data Obtained in Measuring the Stress Optical Coefficients,-of Soda-Alumina-Silica Glasses
Ay, in Degrees
Calculated Stress
Optical Coefficient,
C, in Brewsters
Average Stress
Optical Coefficient,
C, in Brewsters
Standard Deviation, s
2.1683
2.1375
2.1746
2.1477
2.1770
2.6290
2.6065
2.6367
2.6041
2.6396
2.6232
0.0150
2.2875
2.2780
2.2684
2.2838
2.3150
2.8050
2.7950
2.7811
2.8000
2.7910
2.7944
0.0082
2.5022
2.5324
2.5066
2.4901
2.5114
2.9427
2.9782
2.9479
2.9238
2.9535
2.9492
0.0176
2.4413
2.4463
2.4540
2.4320
2.4270
2.9792
2.9623
2.9948
2.9680
2.9618
2.9732
0.0125
Table 9--Continued
Glass
Ay/ in Degrees
AS
2.6805
2.6708
2.6958
2.6842
2.6697
A6
A7
Calculated Stress
Optical Coefficient,
C, in Brewsters
Average Stress
Optical Coefficient,
C, in Brewsters
Standard Deviation, s
2.9939
2.9831 '
3.0110
2.9980
2.9820
2.9936
0.0106
2.4275
2.3538
.2.3808
2.3283
2.4067
2.8510
2.7926
2.7962
2.7623
2.8266
2.8057.
0.0340
2.2988
2.4088
2.3488
2.4317
2.3275
2.6218
2.6671
2.6789
2.6328
2.6545
2.6510
-■
0.0236
<!
Table 10.
Data Obtained in Measuring the Stress Optical Coefficients of Glasses in the D Field of
the Soda-Titania-Silica Ternary System
Calculated Stress
Optical Coefficient,
C, in Brewsters
Average Stress
Optical Coefficient,
C , in Brewsters
Standard Deviation, s
.Glass
Ay, in Degrees
D1
2.4318
2.4605
2.4675
2.4390
2.4813
2.8292
2.8318
2.8398
2.8376
2.8557
2.8388
0.0104
2.2003
2.2200
2.2000
2.2129
2.2336
2.6500
2.6597
2.6495
2.6651
2.6759
2.6600
0.0111
2.4091
2.4318
2.3859
2.4493
2.4275
2.9316
2.9288
2.9034
2.9497
2.9541
2.9335
0.0247
2.3175
2.2850
2.3100
2.3005
2.3175
2.7371
2.7325
2.7283
2.7511
2.7371
2.7372
0.0086
D2
D3
D4
'-j
00
Table 11.
Data Obtained in Measuring the Stress Optical Coefficients of Glasses in the B Field of
the Soda-Titania-Silica Ternary System
Calculated Stress
Optical Coefficient,
C, in Brewsters
Glass
Ay, in Degrees
El
2.2820
2.2775
2.3188
2.2838
2.3110
2.6572
2.6842
2.7000
2.6915
2.6909
2.5008
2.4763
2.4788
2.4688
2.4838
2.9167
2.8909
2.8793
2.9132
2.5820
2.5963
2.5663
2.5713
2.5563
2.2863
2.2913
2.2700
2.3750
2.3700
E2
E3
E4
Average Stress
Optical Coefficient,
C, in Brewsters
Standard Deviation, s
2.6848
0.0164
2.8993
0.0156
2.9903
3.0068
2.9720
2.9988
2.9813
2.9898
0.0138
2.7448
2.7508
2.7253
2.7363
2.7306
2.7376
0,0107
2.8966
CHAPTER 5
DATA ANALYSIS
In the initial stages of this investigation, a fully annealed,
relatively homogeneous glass sample was prepared and placed under a
uniform compressive stress.
Linearly polarized light was then im
pinged on the sample, and the birefringence of the sample ascertained,
by measuring the retardation of the ordinary behind the extraordinary
ray.
This retardation. Ay degrees, was tabulated for each sample in
the preceding chapter.
The calculation and analysis of stress
optical coefficients from Ay values is examined below.
5.1
Calculation of Stress Optical Coefficients
From the theory of photoelasticity,
r
=
CTd
(7)
r
=
retardation of the ordinary behind the
extraordinary fay in angstroms
C
=
stress optical coefficient in brewsters
T
=
stress in pounds per square inch
d
=
thickness of, glass traversed by light
in inches
=
(Aw)/bd
Letting,
T
(12)
80
81
Aw
= weight increment in pounds
d
= thickness of glass traversed by light
in inches
b
= width of glass perpendicular
path in inches (Fig. 33)
to light
And substituting Equation (12) into Equation (7),
Ar
=
[1.752(Aw)Cd]/[bd]
(13)
Ar
=
change in retardation in angstroms
with addition of Aw pounds
1.752
=
conversion factor for the units
employed
The retardation may also be expressed in terms of the angle through
which the plane of polarization is rotated in passing through the
stressed glass and compensator:
Ar
=
Equating Equations
[2(Ay)A]/360.0
(14)
2 (Ay)
=
twice the rotation of the analyzer in
degrees of arc
A
=
wavelength of the light in angstroms
(13) and (14) and solving for the stress optical
coefficient, C,
C
= . [2b(A^)A]/[1.752(Aw)360.0]
Since, in this study A =
5461 angstroms and Aw
(15)
= 5.507 pounds.
82
Glass Specimen
Elliptically
Polarized
Lights
Linearly
Polarized
Light^
J-----
b equals about one centimeter
d equals about one centimeter
Figure 33.
Definition of the "bM and "d" dimensions of the glass
specimens.
83
■ C
=
3.1445 (Ay) b
The stress optical coefficient for each specimen was calculated from
Equation (16).
This calculation procedure, which duplicates that em
ployed by Waxier and Napolitano (1957), was used for the below reason.
Equation (16) may be written.
Ay
=
Q(Aw)
Q
=
(17)
the slope of a straight line
I
Increments of rotation,5 Ay degrees (2.430°, 2.426°, etc.) were
considered in Equation (16) in place of accumulated total rotations
(2.430°, 4.856°, etc.) because for a glass of given composition, the
measurements performed at a specific load were all made on the same
sample.
As a result, errors of an accumulated rotation would have
been cumulative.
Hence, as shown in Table 8, the correct least
squares estimate of slope Q, Ay degrees, was determined by averaging
the five Ay values.
Averaging the eight values recorded for each load to obtain
a retardation, y degrees, also reduced errors in optical measurements.
Waxier and Napolitano (1957) noted that, statistical analysis showed
'
■
'
•. .
this reduced error to be negligible compared to errors resulting from
the method of applying a uniform compressive stress to the sample.
5.2
Statistical Analysis
,
To give an indication of the data's precision,
(1) the ■
standard deviation of the average stress optical coefficient, C,
84
was evaluated, and (2) the "t" test was employed to calculate a 95
percent confidence interval for the mean stress optical coefficient,
C, of each glass.
5.2.1
Standard Deviation
The mean stress optical coefficient, C, of each glass
specimen was determined:
C
=
(XCy/n
=
(C1 + C 2 + C3 + ...)/n
C.
=
a measured value of the stress optical
coefficient
n
=
number of C. values summed
i
(18)
Then the standard deviation, s, was:
s
=
[ZCCh - C)2/(n - I)]0 '5
(19)
Tables 12-14 list the calculated standard deviations.
5.2.2
Student's "t" Test
If
the mean stress optical coefficient, G, is assumed
independent of the standard deviation, s,
then the "t"statistic
distributed as Student's "t" with (n - 1)
degrees of freedom (Johnson
and Jackson 1959).
"t"
is
The "t" statistic is:
=
[(n°-5)(C - u)]/s
C
=
sample mean
u
=
population mean
n
=
number of samples
(20)
85
Table 12.
Glass
Statistical Data on Glasses in the Nepheline and Corundum
Phase Fields of the Soda-Alumina-Silica Ternary System
Average Stress
Optical Coefficient,
(SOC) G, in Brewsters
Standard
Deviation,
s, of SOC
95% Confidence Interval
of SOC Determined Using
Student's "t" Test
A1
2.6232
0.0150
2.6097 to 2.6367
A2
2.7944
0.0082
2.7870 to 2.8018
A3
2.9492
0.0176
2.9333 to 2.9651
A4
2.9732
0.0125
2.9619 to 2.9845
AS
2.9936
0.0106
2.9840 to 3.0032
A6
2.8057
0.0340
2.7750 to 2.8364
A7
2.6510
0.0236
2.6274 to 2.6746
-n
86
Table 13.
Statistical Data on Glasses in the D Phase Field of the
Soda-Titania-Silica Ternary System
Average Stress
Optical Coefficient,
(SOC) C, in Brewsters
Standard
Deviation,
s, of SOC
D1
2.8388
0.0104
2.8294
to 2.8482
D2
2.6600
0.0111 '
2.6500
to 2.6700
D3
2.9335
0.0247
2.9112
to 2.9558
D4
2.7372
0.0086
2.7295
to 2.7449
Glass
95% Confidence Interval
of SOC Determined Using
Student's "t" Test
87
Table 14.
Glass
Statistical Data on Glasses in the E Phase Field of the
Soda-Titania-Silica Ternary System
Average Stress
Optical Coefficient,
(SOC) C, in Brewsters
Standard
Deviation,
s, of SOC
95% Confidence Interval
of SOC Determined Using
Student's "t" Test
El
2.6848
0.0164
2.6700 to 2.6996
E2
2.8993
0.0156
2.8852 to 2.9134
E3
2.9898
0.0138
2.9774 to 3.0022
E4
2.7376
0.0107
2.7280 to 2.7472
88
s :. =
standard deviation
The probability of "t" lying between two values, t^ and t^ may then
be expressed:
P{t1 < (C - u)/(s/(n0 '5) < t 2>
=
1 - a
W
The "t" distribution is symmetrical with its maximum ordinate at
the center; hence, the shortest confidence interval would be obtained
by allowing,
(Xj =
h
=
(%2
a/2 or
(22)
= -t2
(23)
Solving Equation (21) for u produces the confidence limits:
P{[C - (t2s/n°‘5)] < u < [C >
P
=
(t1s/n0 '5)]} = 1 - a
(24)
probability of the interval, {}
The level of significance, a, was, as is usually the case, chosen to
equal 0.05.
This meant there was a 95 per cent probability that the
mean, C, would be within the calculated interval.
The 95 per cent confidence interval of each mean stress optical
coefficient, C, is tabulated in Tables 12-14.
5.5
The
glasses (1)
Linear Regression Analysis
stress optical coefficients of the
in
the
D
soda-titania-silica
phase field, (2)intheE
phase field and(3)
in
both the D and E phase fields, were submitted to the Control Data
6400 computer for linear regression analysis (Appendix B ) .
The computer utilized a standard catalogue program which
solved the matrix AX = Y by searching for the largest element in a
pivotal column.
This procedure, called the Gauss-Jordan numerical
analytical technique, produced coefficients A, B and C for the
equation,
C
=
A Si02 + B Ti02 + C Na20
C
=
(25)
mean stress optical coefficient in brewsters
SiC>2 , Ti02 , Na20
A, B, C
=
=
respective oxide components
in mole fractions
coefficients of proportionality
calculated by the computer
The five commercially melted glasses in the nepheline and
corundum phase fields of the soda-alumina-silica ternary system
had a constant silica content of 0.62 mole fraction, and the linear
regression program could not be applied.
Instead, a Hewlett Packard
9100B computer was programmed to calculate the equation of a line by
the least mean squares method.
C
The equation was of the form:
=
A + B A120 3
(26)
C
=
mean stress optical coefficient in brewsters
A, B
= constants of proportionality calculated by
the computer
90
AlgOg
=
mole fraction of aluminum oxide
Soda-alumina-silica glasses A6 and A7 had variable silica content,
and were submitted with Al, A2 and A3, which were also glasses in
the nepheline phase field, for linear regression analysis.
Finally,
all seven soda-alumina-soda glasses were subjected to the GaussJordan numerical technique.
The equations obtained for each glass phase field and the
measured versus calculated stress optical coefficients are
presented in Tables 15-20.
91
Table 15.
Glass
A1
A2
A3
A3
Average Stress Optical Coefficient Values for Commercial
Laboratory Melted Glasses in the Nepheline and Corundum
Primary Phase Fields of the Soda-Alumina-Silica Ternary
System
Measured
SOC
Calculated
SOC
Difference
2.6396
2.6259*
0.0137
2.6041
2.6259
0.0218
2.6367
2.6259
0.0108
2.6296
2.6259
0.0037
2.6065
2.6259
0.0194
2.8050
2.7889*
0.0161
2.7950
2.7889
0.0061
2.7811
2.7889
0.0078
2.8000
2.7889
0.0111
2.7910
2.7889
0.0021
2.9535
2.9519*
0.0016
2.9427
2.9519
0.0092
2.9782
2.9519
0.0263
2.9492
2.9519
0.0027
2.9238
2.9519
0.0281
2.9535
2.9498**
0.0037
2.9427
2.9498
0.0071
2.9782
2.9498
0.0284
2.9492
2.9498
0.0006
2.9238
2.9498
0.0260
(A)
92
Table 15--Continued
Measured
SOC
Glass
A4
A5
Calculated.
SOC
Difference
(A)
2.9792
2.9720**
0.0072
2.9623
2.9720
0.0097
2.9948
2.9720
0.0228
2.9680
2.9720
0.0040
2.9618
2.9720
0.0102
2.9939 "
2.9942**
0.0003
2.9831
. 2.9942
0.0111
3.0110
2.9942
0.0168
2.9980
2.9942
0.0038
2.9820
2.9942
0.0122
Standard Error = 0.0145
* Calculated by Least Mean Squares Method from the equation:
SOC
=
2.177714 + 4.074750 A l ^
**Calculated by Least Mean Squares Method from the equation:
SOC
=
2.668600 + 1.480000 A1„0„
2 3
93
Table 16.
Average Stress Optical Coefficient Values for Glasses in
the Nepheline Primary Phase Field of the Soda-AluminaSilica Ternary System
Measured
SOC
Calculated
SOC*
Difference
(A)
Al
2.6232
2.6311
0.0079
A2
2.7944
2.8070
0.0126
A3
2.9492
2.9476
0.0016
A6
2.8057
2.7964
0.0093
A7
2.6510
2.6417
0.0093
Glass
Standard Error = 0.0099
*Calculated by linear regression analysis computer program, from the
equation:
SOC
=
3.25921 Si02 + 4.19831 A l ^
+ 0.68054 Na^O
94
Table 17.
Average Stress Optical Coefficient Values for Glasses in
the Nepheline- and Corundum Primary Phase Fields of the
Soda-Alumina-Silica Ternary System
Measured
SOC
Calculated
SOC*
Difference
(A)
A1
2.6232
2.6414
0.0182
A2
2.7944
2.7981
0.0037
A3
2.9492
2.9234
0.0258
A4
2.9732
2.9705
0.0027
AS
2.9936
3.0175
0.0239
A6
2.8057
2.7972
0.0085
A7
.2.6510
2.6424
Glass
1
0.0086
Standard Error = 0.0170
*Calculated by linear regression analysis computer program, from the
equation:
SOC
=
3.30796 Si02 + 3.86311 A l ^
+ 0.72929 Na20
95
Table 18.
Glass
D1
D2
D3
D4
Average Stress Optical Coefficient Values for Glasses in
the D Primary Phase Field of the Soda-Titania-Silica
Ternary System
Measured
SOC
Calculated
SOC*
Difference
(A)
2.8376
2.8351
0.0025
2.8557
2.8351
0.0206
2.8292
2.8351
0.0059
2.8318
2.8351
0.0033
2.8398
2.8351
0.0047
2.6651
2.6595
0.0056 .
2.6759
2.6595
0.0164
2.6500
2.6595
0.0095
2.6597
2.6595
0.0002
2.6495
2.6595
0.0100
2.9034
2.9351
0.0317
2.9497
2.9351
0.0146
2.9541
2.9351
0.0190
2.9316
2.9351
0.0035
2.9288
2.9351
0.006:3
2.7371
2.7399
0.0028
2.7325
2.7399
0.0074
2.7283
2.7399
0.0116
2.7511
2.7399
0.0111
2.7371
2.7399
0.0028
..
Standard Error = 0.0125
*Calculated by linear regression analysis computer program, from the
equation:
SOC = 3.23707 Si0o + 5.23850 TiO_ - 0.27407 Nao0
A
A
A
.
96
Table 19.
Glass
El
E2
E3
■ E4,
Average Stress Optical Coefficient Values for Glasses in
the E Primary Phase Field of the Soda-Titania-Silica
Ternary System
Measured
SOC
Calculated
SOC*
Difference
(A)
2.6572
2.7054
0.0482
2.6842
2.7054
0.0212
2.7000
2.7054
0.0054
2.6915
2.7054
0.0139
2.6909
2.7054
0.0145
2.9167
2.8580
0.0587
2.8966
2.8580
0.0386
2.8909
2.8580
0.0329
2.8793
2.8580
0.0213
2.9132
2.8580
0.0552
2.9903
3.0105
0.0202
3.0068
3.0105
0.0037
2.9720
3.0105
0.0385
2.9988
2.0105
0.0117
2.9813
3.0105
0.0292
2.7448
2.7376
0.0072
2.7508
2.7376
0.0132
2.7253
2.7376
0.0123
2.7363
2.7376
0.0013
2.7306
2,7376
0.0070
/"Calculated by linear regression analysis computer program, from the
equation:
SOC = 2.28800 Si02 + 5.33880 Ti02 + 1.64560 Na20
97
Table 20.
Glass
D1
D2
D3
04
Average Stress 'Optical Coefficient Values, for Glasses in
the D and E Primary Phase Fields of the Soda-TitaniaSilica Ternary System
Measured
SOC
Calculated
SOC*
Difference
(A)
2.8376
2.8065
0.0311
2.8557
2.8065
0.0492
2.8292
2.8065
0,0277
2.8318
2.8065
0.0253
2.8398
2.8065
0.0333
2.6651
2.6828
0.0177
2.6759
2.6828
0.0069
2.6500
2.6828
0.0328
2.6597
2.6828
0.0231
2.6495
2.6828
0.0333
2.9034
2.9474
0.0440
2.9497
2.9474
0.0023
2.9541
2.9474
0.0067
2.9316
2.9474
- 0.0158
2.9288
2.9474
0.0186
2.7371
2.6972
0.0399
. 2.7325
2.6972
0.0353
2.7283
2.6972
0.0311
2.7511
2.6972
0.0539
2.7371
2.6972
0.0399
98
Table 20--Continued
Glass
Measured
SOC
Calculated
’ SOC*
Difference
2.6572
2.7002
0.0430
2.6842
2.7002
0.0160
2.7000
2.7002
0.0002
2.6915
2.7002
0.0087
2.6909
2.7002
0.0093
2.9167
2.8411
0.0756
2.8966
2.8411
0.0555
2.8909
2.8411
0.0498
2.8793
2.8411
0.0382
2.9132
2.8411
0.0721
2.9903
2.9821
0.0082
3.0068
2.9821
0.0247
2.9720
2.9821
0.0101
2.9988
2.9821
0,0167
2.9813
2.9821
0.0008
2.7448
2.8238
0.0790
2.7508
2.8238
0.0730
2.7253
2.8238
0.0985
2.7363
2.8238
0.0875
2.7306
2.8238
0.0932
El
E2
E3
E4
(A) .
Standard Error = 0.0449
^Calculated by linear regression analysis computer program, from the
equation:
SOC
=
2.87805 Si09 + 5.69731 TiO
+ 0.40564 Na 0
CHAPTER 6
RESULTS AND DISCUSSION
Babcock (1968, 1969) denoted two terms describing, the structur
al characteristics of glass.
They were (1) the "substructure," which
provided the foundation or framework of glasses and (2) the "microstructure, ". which related to structural details, as between different
types of substructures.
1.
Babcock (1968, 1969) further postulated that,
Each primary crystallization phase of a glass had a unique
substructure.
2.
Some glass properties were principally determined by these
substructures.
3.
Silicate glasses whould be classified in terms of their
substructures or "silica frameworks."
From the above premises,
(1) each primary phase field correlated with
a unique substructure and (2) a substructure was, by its definition,
directly affected by composition changes; therefore, evidence of the
existence of substructures could be given by proving that some glass
properties had a typical variation with composition in primary
crystallization phases.
Such evidence was presented when Babcock
(1968, 1969) ahd Georoff (1972) showed refractive index, specific
volume, fluidity and Knoop hardness varying linearly with composition
100
in primary phase fields of a number of silicate glasses (Figs. 20, 21).
As an extension of these results, this study was initiated with the
objective of discovering whether the stress optical coefficient also
varied linearly with composition in primary crystallization phase
fields.
6.1
Initial Investigation
Before preparing a number of glasses, it was desired to
obtain some indication of whether birefringence would vary linearly
with composition in primary phase fields.
For this reason, the
preliminary step was to measure the stress optical coefficients of
five commercially melted glasses from the corundum and nepheline
phase fields of the soda-alumina-silica ternary system.
The five
glasses were characterized by a constant silica content of 0.62
mole fraction.
When birefringence was graphed versus the alumina content of
the five samples (Fig. 34), there was clearly an inversion point at
the phase boundary between the corundum and nepheline phase fields.
Both refractive index (Fig. 35) and Knoop hardness (Fig. 36) had an
equivalent inversion point for the same five soda-alumina-silica
glasses.
Because these two glass properties also proved to linearly
vary with composition in primary phase fields, a m o r e .extensive
examination of composition's affect on birefringence was undertaken.
This further examination involved (1) melting and annealing
glasses,
(2) cutting, grinding and polishing specimens,
(3) measuring
101
3.00
2.95
C(brewsters)
2.90
2.85
AUO
N a0O A I00-2SiO-
NEPHELINE
CORUNDUM
2.80
2.75
2.70
2.65
AloO^tmole
Figure 34.
fraction)
Stress optical coefficient, C, versus mole fraction of
aluminum oxide for the five commercial laboratory melted
glasses in the nepheline and corundum phase fields of
the soda-alumina-silica ternary system. -- Silica (Si02)
content constant (0.62 mole fraction).
102
1.507
.506
1.505
1.504
Z
1.503
1.502
1.501
N a 0 O A I 0O v 2 S iO -
AUO
NEPHELINE
CORUNDUM
1.500
AUO *! (mole
Figure 35.
fraction)
Index of refraction, N, versus mole fraction of aluminum
oxide for the five commercial laboratory melted glasses
in the nepheline and corundum phase fields of the sodaalumina-silica ternary system after Babcock (1968). -Silica (SiO^) content constant (0.62 mole fraction).
103
510
505
500
CN
E
495
i
42
490
Z
z
485
480
N o nO-AUO«v2SiO
CORUNDUM
NEPHELINE
475
I
.10
.12
.14
.16
AlgOg (niole
Figure 36.
.18
.20
.22
fractio n )
Knoop hardness number, K.H.N., versus mole fraction of
aluminum oxide for the five commercial laboratory melted
glasses in the nepheline and corundum phase fields of
the soda-alumina-silica ternary system after Georoff
(1972). -- Silica (SiO^) content constant (0.62 mole
fraction).
104
the stress optical coefficients of the glasses,
(4) statistical
analysis of data and (5) linear regression analysis to resolve
whether the data's variation with composition could be described
by the equation of a line. .
6.2
Preparation of Glass Samples
The greater the homogeneity
of a sample, the
the measured stress optical coefficient of the glass.
more realistic
Attaining
homogeneous glass samples was the most difficult task of the study.
6.2.1
Melting
The soda-titania-silica glasses containing lesser amounts
of titania had fluid or "easy" melts.
As the proportion of titania
in the glasses increased, the melts acquired a water-like consistency,
or were, in glass terminology, "fine."
melts, seeds (bubbles) were minimal.
Due to the fluidity of the
The two soda-alumina-silica
glasses (A6 and A7) were more viscous and required twenty to thirty
hours in the furnace to disperse bubbles versus three to twelve hours
for the soda-titania-silica glasses.
Volatilization
atmosphere resulted in
of soda from the melt surface
intothe furnace
a thin layer with a composition deviating from
the remainder of the molten glass.
This layer gradually meshed into
the entire melt as it was poured, producing composition gradients or
streaks throughout the sample.
Stirring with a platinum rod
(1) introduced bubbles and (2) distributed the volatilization layer
throughout the melt as streaks, and after allowing the bubbles to
105
disperse and time enough for diffusion to re-homogenize the glass, a
new surface layer had formed.
The most successful procedure in
minimizing streaks was to take a platinum rod just before pouring
and push this surface layer tip against one half of the platinum
crucible and then pour using the lip of the other half of the crucible.
Other solutions to the problem of volatilization would be (1) melting
in a covered crucible or,
(2) taking a sample, as did Waxier and Napol-
itano (1957), from a 1000-2000 pound melt.
6.2.2
'
Annealing
Cooling the glasses at 2.5°C per hour (Hamilton and Cleek
1958) through the principal annealing range left slight thermal strain
around the edge of the disks that was visible with the polariscope.
Waxier and Napolitano (1957) cooled their samples at a rate of 10C per
hour through the transformation region.
If a more accurate value of
the stress optical coefficient (in the third significant figure) is
desired and time permits, a 1°C per hour cooling rate through the
transformation range is recommended.
There were no instances, of phase separations, and none of
the glasses was hygroscopic.
6.2.3
Grinding and Polishing
The grinding and polishing criteria noted in Figures 24 and
25 appeared adequate.
Any effect upon the measurements due to
surface damage incurred from polishing was not observed by the author.
■ ■
'
This topic was not discussed in the. papers listed in the bibliography.
(
6.3
Measurement of the Stress Optical Coefficient
The stress optic values calculated for each glass are listed
in Tables 9-11.
Birefringence tended to increase with increasing
mole fractions of silica, titania and alumina, and decrease with
increasing mole fractions of soda.
It is of note that the stress optical, coefficient was
measured using light with a wavelength of 5461 angstroms.
How
the stress optical coefficient varies with the wavelength of light
employed, or how it "disperses" is not known.
This would be an
excellent topic for a dissertation.
The accuracy of the measurements could be improved as follows
1.
When the Babinet compensator was set as a quarter wave
plate (Fig. 31), the minimum light intensity or extinction
position was visually noted.
Determination of the minimum
light intensity with a photomultiplier and oscilloscope
would be more accurate.
2.' In defining the optic axes and crossing the nicols, the
first polarizer was adjusted by hand.
Mounting it in a
rotatable circle divider as was the analyzer would increase
the reliability of the data obtained.
3.
For a given load the extinction position was located by
rotating the analyzer until the horizontal line on the
..
oscilloscope screen had dropped to its lowest level.
this minimum point, the line would not move for four
At.
107
to eight turns (0.35 to 0.70 degrees) of the fine adjustment knob
of the rotatable circle divider.
Although centering the reading
within these limits was attempted, this was considered a major source
of error in the third significant figure of the stress optical
coefficients obtained.
Further sophistication of the electronic
analysis of the signal, such as with a Faraday coil, would better
identify the extinction position.
Increasing the intensity of light
entering the photomultiplier would also further clarify the line's
minimum position.
6.4
Linear Regression Analysis
The foremost objective of this study was to determine whether
the stress optical coefficient varied linearly with composition in
phase fields.
Toward this end, all data obtained were examined to
see if they could be represented by a linear equation.
6.4.1
Soda-Titania-Silica Glasses
Table 18 lists the measured stress optical coefficients for
the four glasses in the D field along with the stress optic values
calculated by the equation formulated by the computer.
The small
standard error indicates a high probability of the data being linear.
An equivalent result for glasses in the E field is found in Table 19.
Figure 37 is a two dimensional representation of the linear variation
of the stress optical coefficient in the E and D phase fields of the
soda-titania-silica ternary system.
The stress optical lines are
continuous across the phase field boundary.
108
fraction)
3.011
D
FIELD
2.858*
TiOo (mole
2.935
2.835 * /
2.738
2.705
2 -660x
2.740
E
FIELD
V
05
.20
NagO (mole fraction)
Figure 37.
Lines of equal stress optical coefficient in primary
phase fields D (unknown) and E (Na20.Ti02 .Si02)
of the soda-titania-silica ternary system.
109
Table 20 lists the data returned when,
phase field boundaries, both the D and E field
to linear regression analysis.
without regard to
glasses were subjected
The standard error shows that it is
unlikely a linear equation could represent data from both phase
fields.
Stress optic data on D field glasses fell within the D field
indicated in a phase diagram determined by Hamilton of the National
Bureau of Standards (Levin 1964).
Stress optic data on E field
glasses were in the E field outlined on the same diagram.
6.4.2
Soda-Alumina-Silica Glasses
Because glasses A1-A5 had a constant silica content, the
linear equations
method.
(Table 15) were obtained via the least mean squares
The negligible standard error noted in Table 15 implies the
data are linear (Fig. 34).
Glasses A6 and A7 had differing silica content and were
submitted with glasses
A1-A3, which were also in the nepheline field,
for linear regression analysis.
The measured and calculated stress
optical coefficients are listed in Table 16.
Again, the small
standard error suggests that the data are linear.
When, without regard to phase field boundaries, glasses from
the corundum and nepheline phase fields were subjected to linear
regression analysis (Table 17) the large standard error indicated
a linear equation could not simultaneously represent data from
both phase fields..
A two dimensional illustration of the linear variation of
birefringence in the nepheline phase field of the soda-aluminasilica phase field is drawn in Figure 38.
•
'
Ill
CORUNDUM
CARNEGIEITE
MULLITE
NEPHELINE
W
ALBITE
S1O 2 <m ole
Figure 38.
fra c tio n )
Linear variation of stress optical coefficient in the
nepheline phase field of the N a ^ - A ^ O ^ - S i C ^ glass
CHAPTER 7
CONCLUSIONS
This investigation dealt with the determination and analysis
of stress optical coefficients of glasses in two primary crystalli
zation phase fields of both (1) the soda-titania-silica and (2) the
soda-alumina-silica ternary systems.
The following conclusions
resulted:
1.
Each primary crystallization phase field of a silica glass
has a unique substructure.
2.
This substructure is reflected through the relation between
the stress optical coefficients of glasses within the phase
field.
a.
'
The stress optical coefficients vary linearly with
composition in the primary crystallization phase field.
b.
This linear relationship is continuous across phase
boundaries.
3.
Alumina glasses A1-A5 had equivalent silica contents.
For
these glasses, the stress optical coefficient Versus
composition produced an inversion point at the boundary
between the nepheline and corundum phase fields.
was:
112
This point
113
a.
Similar to that observed for both refractive index and
Knoop hardness versus composition for the same glasses,
b.
Further evidence that primary crystallization phase
fields have unique substructures.
Increasing the mole fraction of:
a.
Titania, silica or alumina tended to increase the stress
optical coefficient of a glass.
b.
Soda tended to decrease the stress optical coefficient of
a glass.
APPENDIX A
CHEMICAL ANALYSIS
114
115
Table A - l .
Chemical Analysis of the Raw Glass Making Materials Melted
in this Investigation
Constituents
Per Cent (%)
Silica (Si02)
Quintus Quartz, Special Low Iron Silica
Assay SiC>2
99.8
Loss on ignition
0.1
Alumina, Al^O^
0.04
Iron oxide, Fe^O^
. 0.0038
Calcium oxide, CaO
<0.001
Magnesium oxide, MgO
<0.001
Sodium oxide, Na20
0.02
Potassium oxide, K20
0.01
Sodium Carbonate (Na2CO )
Anhydrous, Granular, Reagent Grade .
99.9
Assay N a ^ O g
0.003
Insoluble matter
Loss on heating at 285°C
v
0.27
Chloride (Cl)
0.001
Nitrogen compounds (as N)
0.0001
Sulfur compounds (as SO^)
0.001
Ammonium hydroxide precipitate
0.008
Arsenic (As)
0.00002
Calcium and magnesium precipitate
0.009
Heavy metals (as Pb)
0.0003
Iron (Fe)
0.0002
Potassium (K)
0.001
Silica (Si02)
0.003
116
Table A-1--Continued
Constituents
Per Cent (%)
Titanium Dioxide (Ti02)
Powder, Reagent Grade
Assay Ti02
99.3
Acid-soluble substances
0.30
Arsenic (As)
0.0002
iron
(Fe)
0.010
Lead
(Pb)
0.002
Loss
on.ignition
0.20
Water-soluble substances
0.15
Zinc
0.01
(Zn)
Aluminum Oxide ( A ^ O ,
Powder, Reagent Grad
Assay A ^ O ^
•
Residue after ignition
Chloride (Cl)
98.5
1.0
,
0.005
Silicate (Si02).
0.05
Sulfate (S04)
0.005
Heavy metals (as Pb)
0.001
Iron (Fe)
0.03
Substances not precipitated
by NH^OH (as sulfates)
0.75
APPENDIX B
COMPUTER PROGRAM USED FOR LINEAR REGRESSION ANALYSIS
OF STRESS OPTIC DATA
117
118
Table B-l.
0003
0003
0003
0003
0003
0003
0003
0007
0015
0015
0017
0021
0022
0035
0035
0040
0043
0045
0047
0051
0053
0054
0056
0061
0064
0071
0106
0106
0107
0113
0115
0125
0125
0131
0133
0143
0143
0145
0147
0150
0152
0161
0164
.
•
Computer Program Used for Linear Regression Analysis for
Glasses in the Sodium-Titanium-Silicate Ternary System
PROGRAM TEST(INPUT,OUTPUT,TAPE60=INPUT,TAPE61=0UTPUT)
COMMON DATA(8,100)
INTEGER FMS(7),FMT(2)
DIMENSION DELT(100)
DIMENSION V(8),A(10,11)
DATA KI/60/,KO/61/
EXTERNAL VSUB
WRITE(KO,95)
READ 2,KK
2 FORMAT(11)
NOCOEF-KK+1
DO 80 K=l,100
DO 20 1=1,100
READ(KI,59)(DATA(KP,I),KP=1,NOCOEF)
59 FORMAT(8F10.0)
IF(EOF,60)100,18
18 IF(DATA(1,I).EQ.O.O) G O T O 25
20
CONTINUE
STOP 123
100 STOP 456
25
1=1-1
DO 30 11=1,110
A(II)=0.0
30
CONTINUE
CALL LSMTX(KK,I ,A,VSUB,KK)
CALL MATEQ(A,KK,A(1,KK+1))
.WRITE(KO,94)(A(KK,KK+1),11=1,NOCOEF)
94 FORMAT(*C0EF=*7FT2.5)
DLTSUM=0.0
N Q = ((KK-2)*12+2)
IF(KK.LE.2)NQ=1
ENCODE(68,89,FMS)NQ
89 FORMAT(57H(* CALC DEP VAR OBSERVED VAR INDEPENDENT
1
VARIABLES* 12,9HX,*DELT*))
WRITE(KO,FMS)
NQ=KK+3
ENCODE(11,93,FMT)NQ
93 FORMAT(3H(IX,12,6HF12.6))
DO 40 11=1,1
CALL VSUB(II,V,KK)
XX=0.0
DO 35 J=1,KK
.
XX=XX+V(J)*A(J,KK+1)
35
CONTINUE
DELT(II)=DATA(1,II)-XX
119
Table B- l .
0170
0172
0213
0216
0220
0226
0233 •
0233
0237
0241
0241
0006
■ 0006
0006
0007
0016
0021
0021
Computer Program Used for Linear Regression Analysis for
Glasses in the Sodium-.Titanium-Silicate Ternary System-Continued
CALL VSUB(II,V,KK)
WRITE(K0,FMT)XX,V(NOCOEF),(V(IK),IK=1,KK),DELT(I1)
DLTSUM=DLTSUM+DELT(II)**2
40
CONTINUE
DLTSUM=SQRT(DLTSUM/(1-1))
WRITE(KO,91)DLTSUM
91 FORMAT(/1X*SQRT(SUM(DELT(I)**2)/(N-l)=*F12.7)
WRITE(K0,95)
80
CONTINUE
95
FORMAT(1H1)
END
SUBROUTINE VSUB(I,V,KJ)
DIMENSION V (10)
COMMON DATA(8,20)
DO 10 J=1,KJ
10 V(J)=DATA(J+1,I)
V(KJ+1)=DATA(1,I)
RETURN
END
120
Table B-T.
Computer Program Used for Linear Regression Analysis for
Glasses in the Sodium-Titanium-Silicate Ternary System-Continued
SUBROUTINE LSMTX(M>N , A >VSUBR,NUMB)
0010
0010
0012
0013
0014
0020
0023
DIMENSION A(10,ll),VEC(10)
C
M IS THE NUMBER OF FUNCTIONS IN THE POLYNOMIAL
C
N IS THE NUMBER OF DATA POINTS INVOLVED
C
A IS THE ARRAY INTO WHICH THE NORMAL EQUATION MATRIX
WILL BE BUILT
C
C
A MUST BE DIMENSIONED (10,11)
M MUST BE LESS THAN OR EQUAL TO 10
C
N MAY BE ANY SIZE LESS THAN 32,767
C
C
C
C
C
C
C
A SUBROUTINE VSUBR(I,V,NUMB) MUST BE PROVIDED BY THE USER
WHERE
I IS THE INDEX OF THE CURRENT DATA POINT
V IS A VECTOR WHICH MUST BE FILLED BY VSUBR WITH
THE I TH VALUES OF THE M FUNCTIONS FOLLOWED BY
THE I TH VALUE OF Y
NUMB IS A NUMBER TRANSMITTED THROUGH THIS ROUTINE
TO VSUBR
C
FIRST ZERO OUT THE MATRIX
- :
C
0025
0026
0037
0041
0042
0054
0057 "
0061
MAUG=M+1
DO 10 1=1,M
DO 5 J=1,MAUG
A(I,J)=0.0
5 CONTINUE
10 CONTINUE
SECOND BUILD UPPER RIGHT MATRIX
DO 25 IDATA=1,N
CALL VSUBR(IDATA,VEC,NUMB)
DO 20 IROW=l,M
DO 15 ICOL=IROW,MAUG
A(IROW,ICOL)=A(IROW,ICOL)+VEC(IROW)*VEC(ICOL)
15 CONTINUE
20 CONTINUE
25 CONTINUE
121
Table B-l.
Computer Program Used for Linear Regression Analysis for
Glasses in the Sodium-Titanium-Silicate Ternary System-Continued
THIRD FILL OUT SYMETRIC MATRIX
0063
0065
0066
0070
0072
0103
0106
0110
0111
MSUB=M-1
DO 35 IR0W=1,MSUB
ICOLS=IROW+l
DO 30 ICQL=ICOLS,M
A(ICOL,IROW)=A(IROW,ICOL)
30 CONTINUE
35 CONTINUE
RETURN
END
:
SUBROUTINE MATEQ(ARAY,ISIZE,ACOL)
C
GAUSS-JORDAN MATRIX REDUCTION ROUTINE
C
SOLVES THE MATRIX EQUATION A X = Y BY GAUSS-JORDAN
TECHNIQUE
USING LARGEST-PIVOT SEARCH THROUGH THE PIVOTAL COLUMN.
C .
C
C
C
C
C
INPUT IS DEFINED AS FOLLOWS —
ARAY - MATRIX TO BE REDUCED
ACOL '- COLUMN VECTOR
ASSOCIATED WITH -ARAYISIZE - ORDER OF MATRIX -ARAYISING - OUTPUT SWITCH - - 0 = MATRIX ISSINGULAR
1 = MATRIX IS NON-SINGULAR
C.
-ARAY- MUST BE DIMENSIONED IN CALLING
PROGRAM AS ARAY
'(10,10)
C
-ACOL- MUST BE DIMENSIONED IN CALLING
PROGRAM AS ACOL
(10)
C
C
PROGRAMMED BY WM. RABKIN, ITEK CORP.
DECEMBER 1965
C
MODIFIED BY J. RANCOURT 6/2.1/67 TO CONSERVE TIME AND
SPACE
0006
DIMENSION A RAY(10,10),ACOL(10)
C
C
0006
0007
SEARCH FOR LARGEST ELEMENT IN PIVOTAL
DIAGONAL AND SWAP ITS.ENTIRE ROW WITH
DO 65 IPIV=1,ISIZE
IF(IPIV-ISIZE) 5,25,5
COLUMN BELOW
THE PIVOTAL ROW.
122
Table B-l.
0010
0012
Computer Program Used for Linear Regression Analysis for
Glasses in the Sodium-Titanium-Silicate Ternary System-Continued
5 IPP=IPIV+1
DO 20 I=IPP,ISIZE
IF(ABSF(ARAY(I,IPIV))-ABSF(ARAY(IPIV,IPIV)))20,20,10
10 DO 15 J=l,ISIZE
TARAY = ARAY(I,J)
ARAY (I ,J) =ARAY (IP IV,J)
ARAY(IPIVjJ)=TARAY
15 CONTINUE
TARAY =ACOL(I)
ACOL(I)=AC0L(IPIV)
ACOL(IPIV) = TARAY
'
20 CONTINUE
0013
0023
0025
0031
0036
0041
0043
0045
0047
0051
C
0054
IF LARGEST PIVOTAL ELEMENT IS ZERO, MATRIX WAS SINGULAR
25 IF(ARAY(IPIV,IPIV)) 30,70,30
C
0057
00.63
0064
0071
0074
IF NON-ZERO, DIVIDE EACH ELEMENT IN PIVOTAL ROW BY PIVOTA
30 PIVOT=ARAY(IPIV,IPIV)
DO 35 J=IPlV, ISIZE
ARAY(IPIV,J)=ARAY(IPIV,J)/PIVOT
35 CONTINUE
ACOL(IPIV)=ACOL(IPIV)/PIVOT
C
REDUCE EACH ROW EXCEPT THE PIVOTAL ROW
0076
oioo
0102
40
0106
0107
45
0111
0122
50
55
0125
0128
0132
0135
60
65
C
0137
0140
DO 60 1=1,ISIZE
IF(I-IPIV) 40,60,40
CPE=ARAY(I,IPIV)
IF(IPIV-ISIZE) 45,55,45
DO 50 J=IPP,ISIZE
ARAY(I,J)=IPP,ISIZE
CONTINUE
CONTINUE
ACOL(I)=ACOL(I)-CPE*ACOL(IPIV)
CONTINUE
CONTINUE
REDUCTION COMPLETE
-RETURN
NON-SINGULARITY SWITCH
ISING = 1
RETURN
C
MATRIX SINGULAR
SWITCH
OR NCOL = 0 AND INVSW = 0-- RETURN ERROR
123
Table B-l.
0140
0142
0144
0145
0146
Computer Program Used for Linear Regression Analysis for
Glasses in the Sodium-Titanium-Silicate Ternary System-Continued
70 DO 75 I=1,ISIZE
ACOL(1)=0.0
75 CONTINUE
RETURN
END
APPENDIX C
EQUIPMENT EMPLOYED IN THE PREPARATION OF GLASS SAMPLES
124
125
Table C-l.
Equipment Employed in Preparing Glass Samples
--- : . . . -
.... ....
Equipment [and the
preparation step
(from Figure 23)
it was used in]
Torsion optical reading
laboratory balance [3.1]
U. S. Stoneware porcelain
ball mill [3.2]
’
Description
Model PL-2.
Operating on the torsion
principle, it had no knife edges and
was insensitive to out-of-level con
ditions.
Air currents and tempera
ture changes had negligible affect on
accuracy.
The capacity was 2000
grams with an optical projection
reading scale of 110 grams. A scale
vernier allowed readings to 0.01
gram.
Model BFO.
12" long x 6" diameter.
Pereny furnace [3.3]
Model IPKS1732*105. The furnace was
lined with high purity-high density
alumina brick. A second layer of
more porous alumina was integrated
with glass wool material for addi
tional insulation.
"Kanthai" heating
elements were employed.
These ele
ments were made of 90% molybdenum
disilicate and 10% ceramic binding
and were highly oxidation resistant.
Above 982°C the elements reacted
with oxygen to form a silicon dioxide
(quartz glass) coating which protect
ed the MoSi.2 from further chemical
attack (Giler 1970).
The furnace
melting space was three cubic feet
with a maximum temperature capa
bility of about 1600°C.
Honeywell radiamatic
head [3.3]
Type RH, Model 569.
The radiamatic ;
head monitored the temperature in the
Pereny furnace.
PYRO optical pyrometer
[3.3]
The optical pyrometer was used to
calibrate the radiamatic head.
126
Table C-l--Continued
Equipment [and the
preparation step
(from Figure 23)
it was used in]
Honeywell electronic 15
inch circular chart
. proportional controller
(ECCPC)[3.3]
Description
Model Y15201116-01-17-(350)-025-00
with 6 tap-20 KVA Model 1985 trans
former and a Model 1561-382 G01
Norbatrol SCR (silicon controlled
rectifier) package.
This unit pro
vided a temperature control from 700
to 1600°C for the Pereny furnace.
The ECCPC provided a temperature re
cord on a circular chart via an ink
pen receiving input from the
radiamatic head.
Fafnir A1 rollers [3.4]
Model RAS 1.
Corning laboratory hot
plate [3.4]
Model PC-35.
12" long x 6" diameter.
Lihdberg heavy-duty rodoverbend furnace [3.7]
Model 11-R0-122412-20.
This elec
trically heated box type furnace was .
used to anneal glass.
It was lined
with insulating brick and block
type insulation.
Base metal resis
tance type elements heated the fur
nace.
Maximum temperature was
1091°C.
Honeywell electronic
strip chart proportional
controller [3.7]
Model CY15301116-01-3-(350)-002-07.
The ESCPC provided temperature con
trol for the annealing furnace.
This
controller used a thermocouple vs.
the radiamatic head employed by the
Pereny furnace.
A "Data Trak" pro
grammer automatically lowered the
annealing temperature at 2.5°C
through the annealing range.
Thermolyne portable
pyrometer [3.7]
Model PM-1K50. The pyrometer was
used to calibrate the thermocouple
in the Lindberg furnace.
127
Table C-l--Continued
Equipment [and the
preparation step
(from Figure 23)
it was used in]
Description
Bausch and Lomb
polariscope [3.8]
Catalogue No. 31-52-62-60.
Leitz-Wetzlar polarizing
microscope [3.9]
Model DIALUX - Pol.
APPENDIX D
EQUIPMENT EMPLOYED IN MEASURING THE STRESS OPTICAL
COEFFICIENT OF A GLASS SAMPLE
128
129
Table D-l. 'Equipment Employed in Measuring the Stress Optical
Coefficient of a Glass
Equipment [and the
identification number
in Figure 26]
Description
GE tungsten strip lamp +
DC power source [i]
GE. lamp: microscope illuminator,
18A/T10/2P-6V, ASA Code EDW,
The
lamp housing was an EG I G Model
590-20.
Lens [1]
Two inch diameter; radius of
curvature was 70 millimeters.
Interference filter [2]
Oriel 2" square interference
filter, blazed for 5461 angstroms.
Product No. G-572-5461.
Pinhole [3]
Two millimeter diameter pinhole.
Oriel Catalog No. B-26-36-03.
Lens [4]
Two inch diameter; radius of
curvature was 94 millimeters.
Polarizer [5]
UV transmitting polarizing GlanThompson prisms, 0.3’ beam devia
tion, wavelength range to 2300
angstroms, 25° field of view,
length to aperture ration of 3.0;
Yardney Razdow Product No. PGT-UV5121. Mounting tubes were Product
No. RMT-PPR-15C.
The calcite
comprising the polarizer was very
soft and extreme care had to be
used in cleaning.
Apparatus for applying
uniform compression to
glass sample [6]
See Table D-2 and Figures D-l and
D-2.
Soliel-Babinet
compensator [7]
Adjustable through four waves;
Yardney Razdow Product No. SBC12Q-UVV.
Polarizer [8]
Same as Polarizer [5]
___ __________ _____ _______________
130
Table D-l--Continued
Equipment [and the
identification number
in Figure 26]
Divided circle rotator
[8]
Description
Precision angle divider, 360°,
120 millimeter outer diameter,
vernier readable to 0.05°;
Yardney Razdow Product No. DCR120-360.
Lens [9]
Two inch diameter; radius of
curvature was 94 millimeters.
Photomultiplier [10]
RCA Model No. IP21. The power
supply was a Keithely Model 245
with a maximum output of 2000
volts.
300 to 600 hundred volts
were used in the operation of
the photomultiplier.
Oscilloscope [ii]
Hewlett Packard Model HP 108A;
time base plug in Model HP
1821A; differential amplifier
plug in Model H05 1801A.
Optical bench
200 centimeters long; Precision
Tool and Instrument Company Ltd.,
Thorton Heath, Surrey, England.
Weights
Plastic bottles, filled with sand
were used as weights in compres
sing the glass sample.
These
weights were accurate to ± 0.25%.
.
131
0.25
1.75
0.25
7.25
2.25
2.375'
Figure D-l.
2.75
Apparatus for aligning and compressing the glass sample.
132
Table D-2.
Part
Number
(Fig.
D-l)
Itemized Description of Apparatus in Figure D-l.
Dimension
Part
.
Length
Material
16'
Steel
(T)
Yoke
(^2^
Pivot Pin
(7)
Guides (2)
0.75" OD; 0.0625"
wall thickness
0.25"
Brass
(7^
Inserts (2)
0.625" OD; 0.125"
wall thickness
0.25"
Steel
(7)
Shaft
0.375" D
4"
Steel
@
Cylinder
1" OD; 0.125" wall
thickness
7"
Steel
@
Window (2)
0.75" wide x 2.25"
high
Ball Bearing
(2 )
0.125" D
Steel
Plates (2)
See Figure D-2
Steel
10J
Glass Spec
imen
1 x 1 x 3 cm
11)
Base
4.5" D to 2" D to
0.75" D
12)
Screws
8)
0.5" wide x 0.. 1875"
thick
(3 at
Steel
0.25" +
0.25" +
2.5" = 3"
0.25" D
Steel
Aluminum
120°)
13)
Table
7.75" high x 14"
wide
8"
Aluminum
14)
Platform
4" wide
16"
Aluminum
is)
Wire
4 feet from yoke to platform
Steel
I
------1
0.519
0.1875"
0.0625
0.0625"
Figure D - 2 .
Detail of steel caps: (?)
in Figure D - l . A piece
of rubber tape or a piece of rubber gasket was cut
out and placed between the cap and the top of the
glass specimen.
Otherwise, small machining ridges
on the steel cap would have fractured the glass.
SELECTED BIBLIOGRAPHY
Adams, L. H . , and E. D. Williamson.
"The Relation Between Birefring
ence and Stress in Various Types of.Glasses," J. Wash. Acad.,
..
9:609-623, 1919.
Babcock, C. L. "Preparation of Laboratory Glass Samples," Methods of
Experimental Physics, ed. K. Lark-Horovitz and V. A. Johnson,
Vol. VI, Solid State Physics.
New York: Academic Press, 1959,
pp. 139-147.
Babcock, C. L. ."Substructures in Silicate Glasses," J. A mer. C e r . Soc.,
51:163-169, 1968.
Babcock, C. L. "Substructure Classification of Silicate Glasses,"
J. Amer. Cer. S o c ., 52:151-153, 1969.
Babcock, C. L . , and A. N. Georoff.
"Relation of Microindentation
Hardness to Glass Composition," J. Amer. Cer. Soc., to be
published in February, 1973.
Bloss, D. F. An Introduction to the Methods of Optical Crystallography.
New York: Holt, Rinehart, and Winston, 1961.
Brewster, D. Phil. Trans., 105:60, 1814. • ("On the Communication of
Doubly Refracting Crystals to Glass, Muriate of Soda, Fluorspar,
and Other Substances by Mechanical Compression and Dilation,"
Phil. Trans. Roy. S o c ., 106:156, 1816.)
Ernsberger, F. M . ' Polarized Light in Glass Research.
PPG Industries, 1970.
Pittsburgh:
Filon, L. N. G. "Preliminary Note on a New Method of Measuring Directly
the Double-Refraction in Strained Glass," R o y . S o c . Proc.,
A79:440-442, 1907.
("Measurements of the Absolute Indices of
Refraction in Strained Glass," Roy. Soc. Proc., 572-579, 1910.)
Filon, L. N. G. "The Exploration of Stress in the Neighbourhood of an
Isotropic Point in an Elastic Plate," Phil. M a g ., 22:128, 1936.
Filon, L. N. G . , and E. G. Coker.
Treatise on Photoelasticity.
London:
Cambridge University Press, 1931.
Frocht, M. M. Photoelasticity, Vol. II.
Wiley and Sons, Inc., 1948.
134
1st ed.
New York:
2nd ed.
John
135
Georoff, A. N.
"An Investigation of Microindentation Hardness as
Related to Glass Composition," Master's Thesis, The University
of Arizona, 1972.
Giler, R. "Molybdenum Disilicate in Glass Melting," Ceramic Age,
May 1970, pp. 22-24.
Goranson, R. W . , and L. H. Adams.
"A Method for the Precise Measure
ment of Optical Path Difference, Especially in Stressed
Glass," J. Franklin Inst., 216:475, 1933.
Halsey, W. P. Collier's Encyclopedia, Vol. "Number to Phrygia."
New York:
Crowell-Collier Publishing Co., 1963, pp. 168171.
Hamilton, W., and B. W. Cleek.
"Properties of Sodium-TitaniumSilicate Glasses," J . R e s . N a t . Bur. Stds., 61:89-94., 1958.
Johnson, P. 0., and R. W. B. Jackson. Modern Statistical Methods:
Descriptive and Inductive.
Chicago:
Rand-McNally and Co.,
1959.
Kerr, J.
"On the Birefringent Action of Strained Glass," Phil. Mag.,
1:453-63, 1888.
Levin, H. L. Phase Diagrams for Ceramists.
Ceramic Society, 1964.
Columbus:
The American
Lillie, H. R . , and H. N. Ritland.
"Fine Annealing of Optical Glasses,"
J. Amer. Cer. Soc., 37:466-473, 1954.
Maxwell, C. "On the Equilibrium of Elastic Solids," Trans. Roy. Soc.
Edin., 20:87.-120, 1852.
McGraw, D. A., and C. L. Babcock.
"Effects of Viscosity and Stress
Level on Rates of Stress Release in Soda-Lime, Potash-Barium
'
and Borosilicate Glasses," J. Amer. Cer. Soc., 42:330-336,
1959.
,
- Morey, G. W. The Properties of Glass.
Corp., 1954.
New York:
Reinhold Publishing
«
Mueller, H.
"Theory of Photoelasticity in Amorphous Solids,"
Physics, 6:179-184, 1935.
Neumann, F. E. "Die Gesetze der Doppelbrechung des Lichts in
Comprimierten oder Ungleichformig Erwarmten Unkrystallinischen
Korpen," Abh. d. Kon. Acad, d., Wissenschaften zu Berlin,
Part 11:1-254, 1841.
136
Pockels, F.
"Uber die Anderung des Optischen Verhaltens Verschiedener
Glaser durch Elastische Deformation," Annalen der Physik, Ser.
IV, Vol. VII, 9:220-223, 1902; 7:745-771, 1902.
Savur, S. R. "On the Stress Optical Coefficients for Direction Ten
sion and Pressure Measured in the Case of Glass," Phil. M a g .,
1:453-463, 1925.
Schaefer, C., and H. Nassenstein.
"Bestimmung der Photoelastichen
Konstanten p und q Optischer Glaser," Naturforsch, A8:90,
1953.
i Van Zee, A. F. and H. M. Noritake.
"Measurement of Stress-Optical
Coefficient and Rate of Stress Release in Commercial SodaLime Glasses," J. Amer. Cer. S o c ., 41:164-175, 1958.
Vedam, K. "Determination of Photoelastic Properties of Glass," Prbc.
Ind. Acad. S c i ., A31:450, 1950.
Waxier, R. M . , and A. Napolitano.
"Relative Stress-Optical Coef
ficients of Some National Bureau of Standards Optical
Glasses," J. Res. Nat. Bur. Stand., 59:121-125, 1957..
Wertheim, G. "Memoire sur la Double Refraction Temporairement
Produite dans les Corps Isotropes, et sur la Relation entre
L'elasticite Mecanique et L'elasticite Optique," Annales de
Ch. et de Phys., Ser. Ill, Vol. XL:156, 1854.
Wood, R. W.
Physical Optics.
I
New York:
The MacMillan Company, 1934.