* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Total heart volume variation throughout the cardiac cycle in humans
Survey
Document related concepts
Remote ischemic conditioning wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Jatene procedure wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Electrocardiography wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
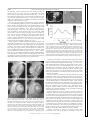
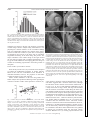
Am J Physiol Heart Circ Physiol 287: H243–H250, 2004. First published March 11, 2004; 10.1152/ajpheart.01125.2003. Total heart volume variation throughout the cardiac cycle in humans Marcus Carlsson,1 Peter Cain,1 Catarina Holmqvist,2 Freddy Stahlberg,3 Stig Lundback,4 and Hakan Arheden1 Departments of 1Clinical Physiology, 2Radiology, and 3Radiophysics, Lund University Hospital, Lund SE-22185; and 4Inovacor, Stockholm SE-18592, Sweden Submitted 25 November 2003; accepted in final form 5 March 2004 cardiac volume. Investigations in humans with the use of computed tomography (15) and MRI in ventilated patients (18) have suggested that a volume variation of 8 –13% may occur between diastole and systole. However, recent work (7) with the use of high-resolution MRI found a lower volume variation of 5% with the largest variation calculated to be at the left side using individual chamber volume measurements at end systole and end diastole (7). These studies confirm the presence, but an unclear extent and timing, of total heart volume variation throughout the cardiac cycle at rest. Additional physiological information may be obtained using measurements of blood flow that are easily obtained using MRI. Therefore, the goals of this study were to investigate the magnitude, timing, and contributors of the total heart volume change during the cardiac cycle in healthy humans with the use of both the MRI-planimetric method as in earlier studies as well as quantitative velocity mapping MRI (21). This will allow the opportunity to explore total heart volume variation from a structural (planimetry) and functional (flow) perspective. The use of both of these principally different techniques in the same subjects gives the possibility to identify whether the volume change is due to a physiological phenomena or a measurement error inherent to the planimetric method (which could explain the large variation between studies). magnetic resonance imaging; left ventricle; output MATERIALS AND METHODS Study Population and Design cardiac function have been studied for centuries (27) with the knowledge acquired providing detailed insights into the fundamental physiology of this organ. Although much of this research has concentrated on the characteristics of the individual cardiac chambers, little is known about total heart volume variation during the cardiac cycle and the respective contributors to this variation. The extent of any volume variation is not just of “academic” concern because it reflects the efficient use of energy by the heart; a large total volume change could result in energy loss through displacement of surrounding tissues or induce a pendular motion of cardiac tissue and blood (11, 12, 14, 20). In 1932, Hamilton and Rompf (11) described a relative constancy of the total heart volume during the cardiac cycle in frogs, turtles, and dogs. They concluded that through the motion of the atrioventricular (AV) plane, the heart was able to pump blood but still maintain the same volume. Subsequent noninvasive investigations in cats (10) and dogs (12, 14) were concordant with this initial finding of a relatively consistent THE CHARACTERISTICS OF HUMAN Address for reprint requests and other correspondence: M. Carlsson, Dept. of Clinical Physiology, Lund University Hospital, Lund SE-22185, Sweden (E-Mail: [email protected]). http://www.ajpheart.org Eight healthy volunteers (26 – 47 yr old, 5 men and 3 women) were examined after approval of the local ethics committee. Subjects underwent cardiac MRI in the supine position. Short-axis gradientecho cine imaging encompassing the entire heart from the base of the atria to the apex of the ventricles was followed by blood flow measurements using velocity mapping in all vessels leading into and out of the heart. Changes in total heart volume throughout the cardiac cycle were calculated directly by planimetry of the gradient-echo cine images and indirectly by subtracting all of the blood flow out of the heart (aorta and pulmonary artery) from all blood flow into the heart (inferior and superior vena cava and the lung veins). Magnetic Resonance Imaging A 1.5-T MRI scanner (Magnetom Vision; Siemens, Erlangen, Germany) with 25 mT/m maximum gradient strength and 600-s gradient ramp time was used to acquire all images. Volumetric imaging. ECG-triggered gradient-echo sequences were used to obtain cine images during end-expiratory apnea (⬃15 s). The number of phases was determined by the R-R interval, resulting in 15–21 images per heart cycle. The typical imaging parameters were The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 0363-6135/04 $5.00 Copyright © 2004 the American Physiological Society H243 Downloaded from http://ajpheart.physiology.org/ by 10.220.33.2 on April 28, 2017 Carlsson, Marcus, Peter Cain, Catarina Holmqvist, Freddy Stahlberg, Stig Lundback, and Hakan Arheden. Total heart volume variation throughout the cardiac cycle in humans. Am J Physiol Heart Circ Physiol 287: H243–H250, 2004. First published March 11, 2004; 10.1152/ajpheart.01125.2003.—Variations in total heart volume (atria plus ventricles) during a cardiac cycle affect efficiency of cardiac pumping. The goals of this study were to confirm the presence, extent, and contributors of total heart volume variation during the cardiac cycle in healthy volunteers with the use of MRI. Eight healthy volunteers were examined by MRI at rest. Changes in total cardiac volume throughout the cardiac cycle were calculated using the following methods: 1) planimetry derived from gradient-echo cine images and 2) flow-sensitive sequences to quantify flow in all vessels leading to and from the heart. The maximum total heart volume diminished during systole by 8.2 ⫾ 0.8% (SEM, range 4.8 –10.6%) measured by method 1 and 8.8 ⫾ 1.0% (SEM, range 5.6 –11.8%) by method 2 with good agreement between the methods [difference according to Bland-Altman analysis ⫺0.6% ⫾ 1.0% (SD), intraclass correlation coefficient ⫽ 0.999]. This decrease in volume is predominantly explained by variation at the midcardiac level at the widest diameter of the heart with a left-sided predominance. In the short axis of the heart, the change of slice volume was proportional to the end-diastolic slice volume. The present study has confirmed the presence of total heart volume variation that predominantly occurs in the region of atrioventricular plane movement and on the left side. The total heart volume variation may relate to the efficiency of energy use by the heart to minimize displacement of surrounding tissue while accounting for the energy required to draw blood into the atria during ventricular systole. H244 TOTAL HEART VOLUME VARIATION IN HUMANS Fig. 1. Volumetric measurements of total cardiac volume. Top: long-axis gradient echo images of the heart at end diastole (A) and end systole (B), showing the four-chamber view of the left ventricle (LV), right ventricle (RV), left atrium (LA), and right atrium (RA). This projection was used to align the short-axis image planes (lines) perpendicular to the septum and parallel to the atrioventricular (AV) plane. Bottom: corresponding short-axis gradient echo images in end diastole (C) and end systole (D). Each short-axis view consisted of 15–21 images, 50 ms apart, covering the cardiac cycle. The dotted line shows how a region of interest (ROI) was drawn around the entire cardiac structure. This was undertaken for every image frame in each imaging plane. AJP-Heart Circ Physiol • VOL Fig. 2. Measurement of blood flow with magnetic resonance imaging. For each anatomic image (A) a corresponding phase image is obtained (B). The anatomic image is used to identify the vessel in which flow shall be measured (in this case, vena cava inferior). A ROI (white oval) representing the vessel and two ROIs (black circle) in nonmoving tissue for background correction are drawn in the anatomic image and copied to the phase image where flow is measured. The grayscale in the phase image is directly proportional to flow velocity in each pixel (scale bar). Darker gray means flow in one direction and lighter gray flow in the opposite direction. The resulting absolute blood flow within the target vessel may then be derived from the combination of velocity values from each pixel (C). Volumetric measurements. A ROI was drawn around the pericardial border (Image software; Scion Image, Scion) of the heart in each image of the cine-loop throughout the heart (Fig. 1, C and D). The ROI included all structures within the pericardium: the atria, ventricles, and the roots of the aorta and pulmonary artery. The area was multiplied by slice thickness to give the volume. The volumes of all slices of the heart were added to identify the total heart volume at each phase during the cardiac cycle. The percentage of total heart volume change was calculated as maximum change divided by total heart volume at end diastole. Flow measurements. Two different evaluation platforms were used (RADGOP; Context Vision, Linköping, Sweden, and Scion Image). Each blood vessel was manually outlined in the anatomic images for each time point with flow calculation undertaken in the corresponding velocity-mapped images. To determine the change in heart volume, the difference between the sums of blood flow into the heart (pulmonary and caval veins) and out of the heart (aorta and pulmonary artery flow) was calculated over the cardiac cycle. Total heart volume change by the flow method divided by total heart volume at end diastole by the volumetric method gives the percentage of total heart volume change for the flow method. Isolated contribution of systole to change in total heart volume. To calculate the total heart volume change that would occur if there were no cardiac inflows to the atria during systole, the combined volume ejected into the aorta and pulmonary artery in systole (the ventricular stroke volumes obtained by the flow method) was divided by the total heart volume at end diastole obtained by the volumetric method. To calculate how large the filling of the heart is during systole, the combined inflows to the heart were divided by the total heart volume at end diastole by the volumetric method. To calculate the percentage of the stroke volumes that is secured by the heart by filling of the atria during ventricular systole, the combined inflows during systole were divided by the stroke volumes obtained by the flow method. Chamber contribution to change in total heart volume. Flow measurements were used to calculate the contribution to the heart 287 • JULY 2004 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.2 on April 28, 2017 the following: 100-ms repetition time (echo sharing resulting in phases every 50 ms), 4.8-ms echo time, 30° flip angle, and 10-mm slice thickness (edge to edge). Short-axis images (Fig. 1, C and D) were taken from cardiac base to ventricular apex perpendicular to the left ventricular longitudinal axis (Fig. 1, A and B). Three long-axis images were obtained in the two-chamber, four-chamber, and the left ventricular aortic-outflow-tract views. Flow imaging. ECG-triggered, flow-sensitive sequences (gradientecho velocity-mapping sequences provided by the manufacturer) were used to measure blood flow in the vessels leading from and to the heart (the aorta, the pulmonary trunk, the superior and inferior caval veins, and the four pulmonary veins). Flow was measured in a perpendicular plane to the vessel. The typical imaging parameters were the following: 30-ms repetition time, 5-ms echo time, 8-mm slice thickness, 150 cm/s velocity encoding, and 36 – 47 images per heart cycle. In the case of aliasing, the study was repeated with 250 cm/s velocity encoding. No images with aliasing were used for analysis. Velocity information was acquired over one or two heart cycles (acquisition time of 3–7 min). Accuracy and precision of this technique has been thoroughly validated (2, 16, 26). Figure 2 shows an example of the velocity mapping technique in an image plane perpendicular to the inferior caval vein. The grayscale values of each pixel in these images (Fig. 2B) are directly proportional to velocity. Thus within a region of interest (ROI), the average blood flow velocity for any given time point throughout the cardiac cycle can be calculated. Knowledge of the measured area will allow calculation of absolute blood flow (average velocity ⫻ area ⫽ average flow) (Fig. 2C). Two ROIs for background correction of field inhomogenities were drawn in stationary tissue at equal length from the vessel investigated, as previously described (2). The average signal intensity of these ROIs in nonmoving tissue were set to represent 0 cm/s. TOTAL HEART VOLUME VARIATION IN HUMANS volume change from the left and the right side of the heart. The net volume difference between inflow and outflow to the left side of the heart (pulmonary veins and aorta) from end diastole to end systole was divided by the total cardiac volume difference from end diastole to end systole. This provided the contribution of the left side (atrium and ventricle) to the total heart volume change in percent. Validation of short-axis measurements and interobserver variability. To validate short-axis measurements [which have proven to be the best plane to determine left ventricular volumes (9, 19) and therefore are commonly used in cardiac MRI], one subject was examined using the frontal, axial, sagittal (5-mm image slice thickness), as well as the short-axis imaging planes (10-mm image slice thickness). The variation in total heart volume was compared between each of these imaging planes. Interobserver variability was performed by two independent observers in all subjects for volumetric measurements and in the first four subjects for flow measurements. Continuous variables are presented as means ⫾ SE and with the range of the variables. The interobserver or method concordance was calculated using the intraclass correlation coefficient (ICC) (25). Bland-Altman analysis (5) and ICC were used to test whether the two methods used to measure changes in total heart volume (volumetric and flow) differed. Paired t-test was used to test whether the changes in apex base length were significant. Pearson’s correlation was used to examine the relationship between relative contribution to total heart volume change and starting slice volume. RESULTS Validation of Short-Axis Measurements and Interobserver Variability Good agreement (ICC 0.91) between imaging planes was observed for total heart volume and its variation and was consistent with previous investigations of interstudy variability (4, 24). Therefore, only one imaging plane (short axis) was used for subsequent subjects. The interobserver variability of planimetry measurements of total heart volume using short-axis images was good (ICC 0.98) and consistent with earlier studies of interobserver agreement of MRI measurement (4, 6, 24). The interobserver variability of flow measurements, ICC ⬎0.99, was also acceptable. The agreement between observers is visually demonstrated by the original data in Fig. 3 where all in- and outflows measured by two observers in one subject is shown. Fig. 3. Flow of all vessels entering and leaving the heart in one subject measured by two independent observers. The outflows of the heart [the aorta (A) and the pulmonary artery (B)] are more pulsatile than the inflows to the heart [cava and pulmonary veins (C–H)], which are more continuous. The graphs show original data from one subject. Note the agreement between the two observers. AJP-Heart Circ Physiol • VOL 287 • JULY 2004 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.2 on April 28, 2017 Statistical Analysis H245 H246 TOTAL HEART VOLUME VARIATION IN HUMANS Table 1. Total heart volume during cardiac cycle and related parameters Subject Sex Age 1 2 3 4 5 6 7 8 Means ⫾ SE F F F M M M M M 37 39 35 26 36 35 47 26 THV Change, %Volumetric THV Change, %Flow THV, ml THV/BSA, ml/m2 Length in ED-ES, % THV Change left, % Ejected Volume/ THV, % Atrial Filling in systole, % Pulse Rate, beats/min 4.8 10.6 6.6 9.1 10.6 6.1 8.8 9.1 8.2⫾0.8 5.6 11.8 6.6 11.8 11.1 5.3 8.9 9.4 8.8⫾1.0 618 584 667 807 715 985 956 891 778⫾55 381 368 389 384 374 442 502 426 408⫾16 ⫺1 0 3 2 1 2 1 ⫺2 0.9⫾0.5 63 62 56 52 66 70 57 62 61⫾2 21 30 21 26 30 18 25 22 24⫾1 73 60 69 55 62 71 64 58 64⫾2 60 57 71 67 53 61 54 53 60⫾2 Fig. 4. Total heart volume during the cardiac cycle in all eight subjects by volumetric and flow measurements. There is a decrease in total heart volume in the first part of the cardiac cycle (systole) and a recovery in volume at the later part of the cardiac cycle (diastole). The change in heart volume by the flow measurements was set to begin at the volume of the heart obtained from the volumetric method. Note the agreement between the two principally different methods in both phase and magnitude. AJP-Heart Circ Physiol • VOL 287 • JULY 2004 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.2 on April 28, 2017 THV change %volumetric, % total heart volume change during the cardiac cycle as measured by gradient echo MRI (volumetric measurement); THV change %flow, % THV change as measured by phase-subtraction, velocity-mapping MRI; THV/BSA, THV normalized to body surface area; length in ED-ES%, mean difference in length of the three long-axis planes from end diastole to end systole divided by the length in end diastole; THV change left%, contribution to total change from the left ventricle and atrium; ejected volume/THV%, volume leaving the heart in systole in aorta and pulmonary artery divided by THV. Atrial filling in systole %, percentage of the stroke volume from the ventricles that is filled into the atria during ventricular systole. TOTAL HEART VOLUME VARIATION IN HUMANS H247 Characteristics of Total Heart Volume Variation During the Cardiac Cycle Volume Variation According to Cardiac Chamber The proportion of total heart volume variation was greater on the left side of the heart, 61 ⫾ 2% (range 52–70%) compared with the right (Table 1). The predominant volume changes were visually observed where the heart was most adjacent to the lungs, whereas the regional changes in volume at the borders to the liver were less and were essentially absent near the thoracic wall (Fig. 8B). Change in Slice Volumes The largest volume change during systole was located where the diameter of the heart was largest, i.e., at the base of the ventricles. This coincided with the region of the AV plane movement (Figs. 1 and 6). The contribution to change in total heart volume from each individual short-axis slice was relative to the size of that slice (r2 ⫽ 0.43) (Fig. 7) resulting in a proportional decrease in volume in all parts of the heart in systole. Notably, the epicardium of the ventricles and the pericardium are opposed (Fig. 8) at end diastole, whereas at end systole, the epicardium of the anterobasal part of the left ventricle is no longer opposed to the pericardium, but instead meets the left atrial appendage. Thus the appendages can be seen to “fill out” the volume loss resulting from ventricular contraction. Longitudinal Shortening of the Heart There was only a minor change in the length of the heart, 0.9 ⫾ 0.5% (range ⫺2 to 3%; P ⫽ 0.07) from apex to base, and the apex of the ventricles was essentially stationary (Table 1). AJP-Heart Circ Physiol • VOL Fig. 5. Temporal difference of cardiac inflow and outflow by flow measurements in one subject. Change in total heart volume was calculated as cumulative volume difference (C; ‚). This was done by mapping inflow and outflow (A) and calculating the cumulative volume (B) as the sum of inflow and outflow at each phase of the cardiac cycle. A: total flow leaving the heart (the sum of the flows of the aorta and the pulmonary artery) is pulsatile and the total flow entering the heart (the sum of the flows of the cava and pulmonary veins) is more continuous. Positive values are inflow and negative values outflow. B: there is a discrepancy in time of the cumulative values of the volume entering and leaving the heart due to the difference in flow patterns described in A. The cumulative curves diverge during systole and converge during diastole. This results in a decrease in total volume in systole that returns to the presystolic volume during diastole. C: volume difference () is negative during systole, meaning that total heart volume (‚) decreases. When the volume difference changes from negative to positive values the total heart volume regains its original value. DISCUSSION This study has shown a variation of ⬃8% (range 5–11%) in total heart volume between diastole and systole in healthy individuals measured noninvasively using cine-MRI. Importantly, both the planimetric and flow-based methods independently show similar results for total heart volume variation in each individual subject and the reproducibility of each of these 287 • JULY 2004 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.2 on April 28, 2017 The overall total heart volume variation during a cardiac cycle did not differ between the volumetric and the flow methods: 8.2 ⫾ 0.8% (range 4.8 –10.6%) versus 8.8 ⫾ 1.0% (range 5.6 –11.8%) (Table 1). ICC was 0.999 and the difference according to Bland-Altman analysis was ⫺0.6 ⫾ 1.0% (SD). The magnitude and time course of the total heart volume variation were essentially similar in all subjects (Fig. 4). It should be noted that the investigated subjects were at rest, with cardiac output of 6.4 ⫾ 0.3 l/min, range 4.7–7.4 l/min; cardiac index 3.4 ⫾ 0.1 l 䡠min⫺1 䡠m⫺2, range 2.9 –3.7 l䡠min⫺1 䡠m⫺2 and low heart rates, range 53–71 beats/min. Examination of the pattern of flow throughout the cardiac cycle, was, however, more complex (Fig. 5). The pulsatile pattern of the outflow in systole and the more continuous, slightly bipolar, inflow both in diastole and systole (Fig. 5A) resulted in a variable flow difference between outflow and inflow over time. In systole, outflow was greater than inflow (decrease in cardiac size), whereas the reverse was true during diastole (Fig. 5C). These findings were reflected across the study population with the proportion of blood ejected during systole exceeding the filling of the atria during systole; 24 ⫾ 1% (range 18 –30%) versus 15 ⫾ 1% (range 13–19%) of the total heart volume (P ⫽ 0.001). During systole, 64 ⫾ 2% (range 58 –73%) of the stroke volume from the ventricles was secured by filling of the atria (Table 1). H248 TOTAL HEART VOLUME VARIATION IN HUMANS techniques was shown to be high. The variation in total heart volume change between subjects likely demonstrates a true physiological variation because of the concordant findings from two independent methods. The variation arises from a decrease in total heart volume in systole due to a discrepancy in blood flow into and out of the heart with no significant longitudinal shortening of the heart during this process. The major contributor to the volume change is the region around the AV plane movement with a leftsided predominance. For any given cardiac MRI short-axis imaging plane, the change in volume was essentially proportional to the starting volume in that plane. Earlier Studies of Total Heart Volume Since the days of Da Vinci (27), researchers have attempted to explain the fundamental properties of cardiac function. Much of this work has concentrated on the mechanics of individual chambers; however, the properties of total heart volume and shape are less well explored. Earlier studies undertaken in this area using animal and human subjects have produced conflicting results regarding the variation of cardiac volume. Some reported no variation (11), Fig. 7. Volume change as a function of slice volume. y-Axis: heart volume change (C) in one slice in percentage of total heart volume change in that subject. x-Axis: heart volume (V) in one slice in percentage of total heart volume at end diastole. The relationship is shown between the relative contribution from a single slice to the total heart volume change (y-axis) and the relative contribution of that slice to the total heart volume (x-axis). The larger a slice is, the more it contributes to total heart volume change. The relationship is essentially proportional. The equation for the regression line is: y ⫽ 1.1x ⫺ 0.75. AJP-Heart Circ Physiol • VOL Fig. 8. Regional differences in total heart volume changes. app, Appendage. Top: short-axis views of the heart at end diastole (A) and end systole (B). The pericardium in end diastole is marked with a dotted line both in A and B. A variation of volume has occurred from diastole to systole where the epicardium no longer opposes the dotted line in B. The level of the short-axis plane shown is chosen where the diameter of the heart is the largest. There is a larger volume change seen at the left-hand side than the right-hand side. At the left-hand side, there is a volume change where the epicardium opposes the pericardium but no volume change where the left atrial appendage opposes the pericardium. Note that at end systole (B) due to AV plane descent the left ventricular (LV) outflow tract can be seen where myocardium was seen at end diastole (A). Bottom: long-axis two-chamber views of the heart at end diastole (C) and end systole (D). The broken line indicates the epicardium of the left ventricle at end diastole and the dotted line the epicardium at end systole. At the basal level of the LV in end systole the epicardial border (dotted line D) does not oppose the pericardium but the left atrial appendage (also seen in B). The same finding can be seen for the right atrial appendage at the right ventricle (B). some reported a variation within the measurement error (14), and others suggested that a variation indeed exists, although the total heart volume variation varies between studies from 5 to 13% (7, 12, 15, 18). In 1932, Hamilton and Rompf (11) did a study of the heart in frogs, turtles, and dogs and concluded that the variation of total heart volume during the heart cycle was minimal and that much of the cardiac output was generated by movement of the AV plane. Subsequent work by Gauer (10) expanded these initial findings by acquiring fluoroscopic ventriculograms of cat hearts and concluded that the “outside dimensions of the heart. . .identical” between systole and diastole. Further studies by Hoffman et al. (12, 15) in dogs were consistent with this observation with a total heart volume variation of 5% that increased with atrial fibrillation. However, Leithner et al. (18) demonstrated in humans a variation of up to 13% of intracavitary volumes but suggested that this could not be considered as total heart volume and therefore was guarded in drawing any conclusions about true total heart volume variation. Bowman et al. (7) recently proposed that the total 287 • JULY 2004 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.2 on April 28, 2017 Fig. 6. Anatomic location of change in total heart volume in a typical subject. The main part of the change in volume between diastole and systole was located at the base of the ventricles i.e., where the diameter of the heart is largest. A typical finding was a decrease in volume in all slices of the heart during ventricular systole. The largest slices, and the largest contribution to total heart volume change, were found at the midcardiac level, at the region of the AV plane movement. TOTAL HEART VOLUME VARIATION IN HUMANS chamber volumes in the pericardium may be less constant than the blood pool content. In the present study, however, we did not find any significant difference between total heart volume variation obtained by flow or planimetric measurements. The variation between subjects in total heart volume change is likely a true physiological finding because two principally different and independent techniques (flow and planimetry) yielded highly concordant results. This intersubject variation may explain the differing results in total heart volume change found in previous studies. Physiological Relevance Further Studies Studies with MRI under dobutamine and/or atropine stress would give the opportunity to tease out the effect on total heart volume variation of higher heart rates and cardiac outputs AJP-Heart Circ Physiol • VOL discussed above. This could give new information on the cardiac energetics in man under different physiological conditions. Limitations The volumetric images in this study were acquired during end-expiratory apnea. This potentially may affect cardiac output, pulse rate, and introduce a potential “valsalva-like” effect on the filling of the cardiac chambers. In addition, the temporal resolution of the volumetric image sequences was 50 ms and thus may be an undersample of the true end-diastolic and end-systolic dimensions of the heart. Despite these potential errors, the flow-based measurements that have a higher temporal resolution (30 – 40 frames per heart cycle) and are acquired during free breathing were in close concordance with the volumetric measurements and therefore suggests that these errors are likely to be small at most. It should be noted that the volumetric images are acquired during 15–19 heartbeats per slice and that the flow images are acquired during 256 heartbeats (or 512 when the images were obtained over two R-R intervals). Other imaging limitations inherent to cardiac MRI were occasionally present during this study, including the “partial volume effect,” which results from a composite image of 1-cm imaging planes. This effect, however, is also likely to be small as demonstrated by the validation of the 1-cm shortaxis slice thickness with 0.5-cm slice thickness measurements in three orthogonal planes. In conclusion, the present study has demonstrated in humans that total cardiac volume, during a full cardiac cycle at low cardiac output and heart rate, diminishes 8% (range 5–11%) during systole and predominantly occurs in the region of the AV plane and on the left side. The total heart volume variation may relate to the efficiency of energy use by the heart to minimize displacement of surrounding tissues while accounting for the energy required to draw blood into the atria during ventricular systole. GRANTS This study was supported by the Swedish Research Council, the Swedish Heart and Lung Foundation, and The Medical Faculty of Lund University. REFERENCES 1. Appleton CP. Hemodynamic determinants of Doppler pulmonary venous flow velocity components: new insights from studies in lightly sedated normal dogs. J Am Coll Cardiol 30: 1562–1574, 1997. 2. Arheden H, Holmqvist C, Thilen U, Hanseus K, Bjorkhem G, Pahlm O, Laurin S, and Stahlberg F. Left-to-right cardiac shunts: comparison of measurements obtained with MR velocity mapping and with radionuclide angiography. Radiology 211: 453– 458, 1999. 3. Bartzokis T, Lee R, Yeoh TK, Grogin H, and Schnittger I. Transesophageal echo-Doppler echocardiographic assessment of pulmonary venous flow patterns. J Am Soc Echocardiogr 4: 457– 464, 1991. 4. Bellenger NG, Davies LC, Francis JM, Coats AJ, and Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2: 271–278, 2000. 5. Bland JM and Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986. 6. Bogaert JG, Bosmans HT, Rademakers FE, Bellon EP, Herregods MC, Verschakelen JA, Van de Werf F, and Marchal GJ. Left ventricular quantification with breath-hold MR imaging: comparison with echocardiography. Magma 3: 5–12, 1995. 7. Bowman AW and Kovacs SJ. Assessment and consequences of the constant-volume attribute of the four-chambered heart. Am J Physiol Heart Circ Physiol 285: H2027–H2033, 2003. 287 • JULY 2004 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.2 on April 28, 2017 A low total heart volume variation will lower the energy required to move surrounding tissues during filling and emptying of the heart (12). Our qualitative visual assessment and quantitative findings (Table 1) have shown that the largest volume variation occurred in the region of the AV plane and slightly more on the left-hand side and is consistent with previously published findings (13). At higher heart rates and cardiac output, the momentum of the blood created by the descent of the AV plane can be used for a more rapid filling of the expanding atria and the reverse motion of the AV plane. Thus at higher heart rates, blood will have a higher momentum, decreasing the requirement of outer volume change in the surrounding tissue, which would save energy. In addition, higher heart rates may result in a lower total heart volume change because of a shortened diastolic phase, and this possibly could make the outflow less pulsatile, and hence, synchronize the in- and outflow (20). This could result in a lesser total heart volume change at higher frequencies. This is in line with the findings of Brecher (8), Nilsson et al. (22), and the conclusions of Gauer (10) that faster and smaller hearts secure 80% of their stroke volume during ventricular systole. In the present study in humans (larger species and therefore larger hearts and slower heart rates) 64% (range 55–73%) of the stroke volumes from both chambers were secured into the atria during systole (reservoir function of both atria). The reservoir function of the left atrium in percentage of the stroke volume of the left ventricle has previously been reported to be 38% as assessed by Doppler echocardiography (23) and 41% as assessed by MRI (17). Thus the reservoir function of the right atrium is higher than the left atrium. This can be visualized in the flow curves as a more bipolar flow in the caval veins with the highest peak in systole and a more continuous flow in the pulmonary veins. In Doppler examination of caval and pulmonary veins, a similar biphasic pattern of blood flow during systole and diastole into the heart as in the present study has been found (3) and also alteration of cardiac filling according to heart rate (1). At an increased heart rate, proportionally greater systolic atrial filling over diastolic flow occurs compared with that which occurs at lower heart rates. These findings with echocardiography are consistent with the previously stated relationship between cardiac volume variation and heart rate, as increased systolic atrial filling would minimize volume variation. H249 H250 TOTAL HEART VOLUME VARIATION IN HUMANS AJP-Heart Circ Physiol • VOL 18. Leithner C, Podolsky A, Globits S, Frank H, Neuhold A, Pidlich J, Schuster E, Staudinger T, Rintelen C, Roggla M, Glogar D, and Frass M. Magnetic-resonance-imaging of the heart during positive end-expiratory pressure ventilation in normal subjects. Crit Care Med 22: 426 – 432, 1994. 19. Longmore DB, Klipstein RH, Underwood SR, Firmin DN, Hounsfield GN, Watanabe M, Bland C, Fox K, Poole-Wilson PA, Rees RS, Denison D, McNeilly AM, and Burman ED. Dimensional accuracy of magnetic resonance in studies of the heart. Lancet 1: 1360 –1362, 1985. 20. Lundback S. Cardiac pumping and function of the ventricular septum. Acta Physiol Scand 127: 8 –101, 1986. 21. Nayler GL, Firmin DN, and Longmore DB. Blood flow imaging by cine magnetic resonance. J Comput Assist Tomogr 10: 715–722, 1986. 22. Nilsson NJ and Kurt K. Stromvolumpulse der herznahen Venen bei verschiedenen Kreislaufzuständen. Zeitschrift Biologie 106: 386 –394, 1954. 23. Prioli A, Marino P, Lanzoni L, and Zardini P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am J Cardiol 82: 756 –761, 1998. 24. Semelka RC, Tomei E, Wagner S, Mayo J, Kondo C, Suzuki J, Caputo GR, and Higgins CB. Normal left ventricular dimensions and function: interstudy reproducibility of measurements with cine MR imaging. Radiology 174: 763–768, 1990. 25. Shrout PE and Fleiss JL. Intraclass correlations– uses in assessing rater reliability. Psychol Bull 86: 420 – 428, 1979. 26. Sondergaard L, Hildebrandt P, Lindvig K, Thomsen C, Stahlberg F, Kassis E, and Henriksen O. Valve area and cardiac output in aortic stenosis: quantification by magnetic resonance velocity mapping. Am Heart J 126: 1156 –1164, 1993. 27. Wandt B. Long-axis contraction of the ventricles: a modern approach, but described already by Leonardo da Vinci. J Am Soc Echocardiogr 13: 699 –706, 2000. 287 • JULY 2004 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.2 on April 28, 2017 8. Brecher GA. Cardiac variations in venous return studied with a new bristle flowmeter. Am J Physiol 176: 423– 430, 1954. 9. Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, Parker RA, Douglas PS, and Manning WJ. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol 35: 477– 484, 2000. 10. Gauer OH. Volume changes of the left ventricle during blood pooling and exercise in the intact animal: their effects on left ventricular performance. Physiol Rev 35: 143–155, 1955. 11. Hamilton WF and Rompf JH. Movement of the base of the ventricle and the relative constancy of the cardiac volume. Am J Physiol 102: 559 –565, 1932. 12. Hoffman EA and Ritman EL. Heart-lung interaction: effect on regional lung air content and total heart volume. Ann Biomed Eng 15: 241–257, 1987. 13. Hoffman EA and Ritman EL. Intracardiac cycle constancy of total heart volume. Dyn Cardiovasc Imaging 1: 199 –205, 1988. 14. Hoffman EA and Ritman EL. Invariant total heart volume in the intact thorax. Am J Physiol Heart Circ Physiol 249: H883–H890, 1985. 15. Hoffman EA, Rumberger J, Dougherty L, Reichek N, and Axel L. A geometric view of cardiac “efficiency” (Abstract). J Am Coll Cardiol 13: 86A, 1989. 16. Hundley WG, Li HF, Hillis LD, Meshack BM, Lange RA, Willard JE, Landau C, and Peshock RM. Quantitation of cardiac output with velocity-encoded, phase-difference magnetic resonance imaging. Am J Cardiol 75: 1250 –1255, 1995. 17. Jarvinen V, Kupari M, Hekali P, and Poutanen VP. Assessment of left atrial volumes and phasic function using cine magnetic resonance imaging in normal subjects. Am J Cardiol 73: 1135–1138, 1994.