* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Adult Congenital Heart Disease Patient
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Electrocardiography wikipedia , lookup
Myocardial infarction wikipedia , lookup
Coronary artery disease wikipedia , lookup
Rheumatic fever wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Aortic stenosis wikipedia , lookup
Artificial heart valve wikipedia , lookup
Cardiac surgery wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Congenital heart defect wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
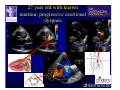
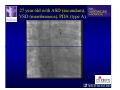
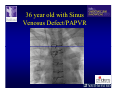
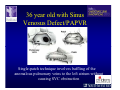
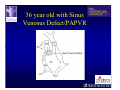
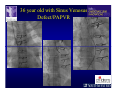
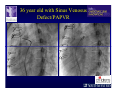
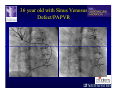
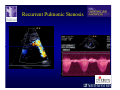
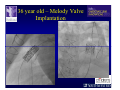
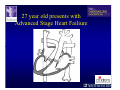
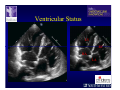
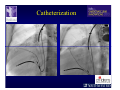
The Adult Congenital Heart Disease Patient Michael Luna, MD Assistant Professor Department of Internal Medicine Interventional Cardiology Adult Congenital Heart Disease Program UT Southwestern Medical Center Disclosures I receive research funding from AstraZeneca. Field of Adult Congenital Heart Disease • Reparative surgery for congenital cardiac lesions has lead to dramatic improvement in survival • ~85% will survive into adulthood • In 2000, 32nd Bethesda Conference reported an estimated 800,000 patients living with some form of ACHD Marelli et al. Circulation. 2007;115:163–72. Warnes et al. J Am Coll Cardiol. 2001;37:1170 –5. Scope of Congenital Heart Disease in the Cath Lab • Diagnostic catheterizations – shunt calculation – pulmonary hypertension – complex cyanotic congenital lesions – angiography • Interventional catheterizations – Shunt lesion occlusions • device occluders • coils • exclusion by covered stent – Vascular stenting • bare metal stents • covered stents • drug-eluting stents – Valve implantation • Melody valve 27 year old with known murmur and progressive exertional dyspnea • G3P3 with uncomplicated deliveries • Delivered third child at our institution • Referred for chest wall echocardiogram 27 year old with known murmur, progressive exertional dyspnea 27 year old with ASD (secundum), VSD (membranous), PDA (type A) 27 year old with ASD (secundum), VSD (membranous), PDA (type A) 27 year old with ASD (secundum), VSD (membranous), PDA (type A) 27 year old with ASD (secundum), VSD (membranous), PDA (type A) 36 year old with PAH associated with CHD • Prior professional ballerina • Severe, progressive exertional dypsnea in late 20s • Diagnosed with PAH and referred to our Pulmonary Hypertension group • Initially felt to be iPAH but sinus venosus defect with anomalous drainage of RUPV/RMPV was detected by cath – – – – Bidirectional shunting (Qp/Qs = 1.5:1) Rp ~12 Wood units Rp decreased to ~6 Wood units with pulmonary vasodilators Referred for surgical repair of the sinus venosus defect/PAPVR 36 year old with Sinus Venosus Defect/PAPVR 36 year old with Sinus Venosus Defect/PAPVR Single-patch technique involves baffling of the anomalous pulmonary veins to the left atrium without causing SVC obstruction 36 year old with Sinus Venosus Defect/PAPVR 36 year old with Sinus Venosus Defect/PAPVR 36 year old with Sinus Venosus Defect/PAPVR 36 year old with Sinus Venosus Defect/PAPVR 36 year old with Sinus Venosus Defect/PAPVR 36 year old, born with severe valvar pulmonic stenosis • Underwent pulmonary valvectomy at 3 years of life • Surgical PVR ~8 years ago due to progressive RV dilation 21 mm Carpentier-Edwards Magna Valve 36 year old, born with severe valvar pulmonic stenosis • • • • • BMI ~56 OSA on CPAP HTN Hypothyroidism DVT/PE history Recurrent Pulmonic Stenosis Therapeutic Options? RVOT Dysfunction • Many congenital cardiac defects require surgical RVOT reconstruction: – placement of a right ventricle (RV) to pulmonary artery (PA) conduit – PV valve replacement with bioprosthesis RVOT Lesions • RVOT/PV stenosis/atresia • Absence of the pulmonary artery • Pulmonary insufficiency Pulmonic Valve Bioprosthesis • Long term performance remains poor • Controversy regarding differential performance of bioprosthetic material Jang et al. European Journal of Cardio-Thoracic Surgery 42 (2012) e1–e8. RV-PA Conduit Outcomes Multiple surgical procedures for conduit replacement or revision is common Ong et al. Am J Cardiol. 2013 Jun 1;111(11):1638-43. Treating Conduit Dysfunction • Surgical conduit replacement/revision • Bioprosthetic valve placement • Percutaneous valve placement Transcatheter Pulmonary Valve Replacement • Allows for pulmonary valve replacement without cardiopulmonary bypass in appropriate patients – – – – RV-PA conduit 16 mm and larger Bioprosthetic valves Anatomy amenable to implant Adequate patient size (22 Fr system) • 30 kg Transcatheter Pulmonary Valve Replacement 36 year old with repaired congenital PS 36 year old – Melody Valve Implantation Severe coronary compression 36 year old – Melody Valve Implantation 27 year old presents with Advanced Stage Heart Failiure • Dextrocardia • L-transposition of the great arteries • Pulmonary atresia with ventricular septal defect – initial palliative shunt creations early in life (bilateral modified Blalock-Taussig shunts Waterston shunt) • Complete repair (age~7): – LVOT reconstruction with a 19 mm aortic homograft – VSD closure – Complicated by complete heart block (dual chamber pacemaker implantation) 27 year old presents with Advanced Stage Heart Failiure Hemodynamic History • Declining Max VO2’s beginning—2002 (32 in 2000 to 22 in 2002) • Clinical heart failure symptoms in—~2007 (LV [subpulmonary] systolic function mildly depressed) RA LV PA PCW RV CI 3/09 7 75/6 17/9 (13) 6 95/9 -- 8/10 21 -- -- -- -- 2.0 10/11 21 77/21 25/15 (18) 13 78/17 1.89 12/12 18 81/19 30/18 (22) 16 78/16 1.74 Ventricular Status LV RV RA LA Catheterization Catheterization Qp = 2.24 L/min -- 1.48 L/min/m^2 Qs = 2.64 L/min -- 1.75 L/min/m^2 Qp/Qs = 0.85:1 Rp (cannot be calculated due to obstruction) Rs = 23.11 Wood units -- 34.86 Wood units-m^2 Conclusions • Adults with all forms of congenital heart disease require longterm follow-up • Patients with complex congenital heart disease require specialized care at a center with established expertise in congenital heart disease