* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Abstract
Survey
Document related concepts
Rett syndrome wikipedia , lookup
History of neuroimaging wikipedia , lookup
Asperger syndrome wikipedia , lookup
Serotonin syndrome wikipedia , lookup
Williams syndrome wikipedia , lookup
Dual consciousness wikipedia , lookup
Marfan syndrome wikipedia , lookup
Down syndrome wikipedia , lookup
Wernicke–Korsakoff syndrome wikipedia , lookup
Guillain–Barré syndrome wikipedia , lookup
Hemiparesis wikipedia , lookup
Werner syndrome wikipedia , lookup
Transcript
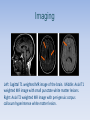
Abstract Although Susac syndrome was first described in 1979, it remains an uncommon diagnosis. Advanced imaging modalities have contributed to the understanding of the pathology involved, however many physicians remain unfamiliar with this disorder. Imaging and autopsy exams suggest Susac syndrome produces microangiopathic changes, the result of a gradual destructive, likely auto-immune process. Abstract Despite many patients having classic MRI findings it remains difficult to diagnose prior to the onset of a patient’s more severe symptoms. These symptoms, seen as a clinical triad, include visual field deficits, sensorineural hearing loss, and encephalopathy. Once diagnosed, treatment regimens are inconsistent throughout the literature with variable outcomes. Below is an atypical presentation, one without characteristic MRI or angiographic changes, but with a significant response to a known therapeutic regimen. Introduction Susac syndrome, also known as retinocochleocerebral vasculopathy, was originally described by John Susac in 1979 and was first characterized by encephalopathy, visual deficits, and sensorineural hearing loss. Currently, clinical evaluation with the support of imaging studies, including fluorescein angiography and magnetic resonance imaging (MRI), are used to make a diagnosis. Introduction Pathognomonic MRI findings include T2-hyperintense deep gray matter and supratentorial lesions involving the corpus callosum, often described as “snowballs,” which may be accompanied by parenchymal and leptomeningeal contrast enhancement. Visual changes on imaging represent pauciinflammatory microangiopathy with infarction, sclerosis, and or fibrin deposition in capillaries and arterioles. Further, audiology examination reveals low frequency hearing loss. While retinal fluorescein angiography shows sporadic segmental retinal arterial occlusions. Introduction Limited clinical case reports exist on Susac syndrome particularly among Neurology, Ophthalmology, Pathology and Radiology. Commonly the age of disease onset appears to be between ages 20 to 40, although there have been reported cases of Susac syndrome in patients as young as 7 and as old as 72 years of age. Also noted is a slight female predominance. Introduction The complex symptoms of this syndrome makes distinguishing Susac syndrome from Multiple Sclerosis (MS) and Acute Disseminated Encephalomyelitis (ADEM) difficult as they have much in common. In all three diseases, symptoms can arise in rapid progression and patients can experience symptom-free periods or remissions. Headaches can be prodromal. Introduction However, unlike MS and ADEM, MRI findings involving leptomeningeal enhancement, deep gray matter, and “snowball” lesions in the central corpus callosum are distinguishing features representative of microangiopathy and not demyelination.1,2 Retinal fluorescein angiography in a Susac patient will classically show non-perfused retinal arterioles and hyperfluorescent walls consistent with retinal artery branch occlusions with normal choroidal circulation which is unlikely to be seen in either MS or ADEM.3 Introduction Despite unknown pathogenesis, there appears to be an autoimmune connection leading to microvasculature changes in the brain, retina, and inner ear due to inflammation and possible endotheliopathy. Anti-Endothelial Cell Antibodies (AECAs) have been found to be present in the disease although significance has yet to be completely determined. It has been theorized that AECAs may prove to be a “diagnostic biomarker.”4 Introduction In addition to the classic retinal, inner ear, and neurological findings, muscle aches and skin lesions can also be present in Susac syndrome. Patient complaints of myalgias as well as rashes, such as livedo reticularis, have been seen in prior cases. Direct immunofluroescence of rashes show sparse granular C3 component and IgA deposition. Biopsies reveal endothelial cell swelling and lymphocytic and histiocytic infiltrates within the upper dermal capillaries and arterioles. Fibrin deposition could also be suggestive of a procoagulopathic state, although this has not been proven.3 Introduction These findings suggest that Susac syndrome may have the systemic manifestations of inflammatory disease.2,4 With suspected auto-immune pathophysiology and a rare triad of symptoms, it is probable that the Rheumatologist will encounter these patients in the future for possible diagnosis as well as management. Clinical Case A forty-four year old Caucasian female presented to Rheumatology seven years after initial symptoms. In 2006, this patient experienced sudden onset of severe vertigo with loss of consciousness while seated during her night shift as an ED nurse. Her past medical history at the time was notable only for migraine headaches. Medications were topiramate at 50mg tablets twice daily and sumatriptan 100mg twice daily as needed. Also, at the time of her symptoms, she was two months post partum after an unremarkable delivery. CT results did not reveal abnormality. Clinical Case Over the next few months she developed daily headaches, confusion, difficulty ambulating, and later tinnitus. She was subsequently seen by ENT and diagnosed with benign paroxysmal positional vertigo and treated unsuccessfully with physical therapy. MRI 18 months later revealed abnormalities suspicious for demyelination and thus multiple sclerosis (MS). Clinical Case Findings on the MRI included many small foci by increased T2 and FLAIR signal within the subcortical and deep white matter bilaterally. One lesion in particular was found near the genu of the corpus callosum. The lesions were nonenhancing. Additional opinions from Neurology determined that these were not consistent with MS. Clinically her symptoms continued to worsen despite a rigorous therapy of ondansetron 4mg tablets every eight hours, famotidine 20mg daily, diazepam 5mg four times daily, levetiracetam 500mg nightly, and clonazepam 0.5mg nightly. Clinical Case The patient was seen nearly seven years after her initial symptoms at the Cleveland Clinic by a Susac specialist. Her cranial nerves, deep tendon reflexes, and mental status examinations were unremarkable, despite a wobbly gait, confusion, and hearing loss. She was seen by Ophthalmology who reported no evidence of retinal vasculopathy on fluorescein angiography. Audiology examination revealed a ten decibel sensorineural hearing loss on the right at high frequencies between 2-8,000 Hz. Clinical Case A repeat MRI revealed few punctuate T1, T2 and FLAIR hyperintensities scattered through the subcortical white matter of the frontal lobes bilaterally with periventricular nonenhancing white matter hyperintensities surrounding, and possibly involving, the corpus callosum. Her cerebral spinal fluid did not reveal oligoclonal bands nor elevated protein. After seven years a clinical diagnosis of Susac syndrome was made despite inconclusive, but suggestive, imaging and angiography studies. Clinical Case The patient received pulse corticosteroids and was placed on prednisone along with mycophenolate mofetil titrated to1000mg twice daily. Although symptoms began to improve, there was a dramatic response once monthly IVIG was added seven weeks later. She remains on IVIG every 4 weeks as well as mycophenolate mofetil and a tapering dose of steroids, currently at 12.5mg daily, to fully control her symptoms. Clinical Timeline Imaging Left: Sagittal T1 weighted MR image of the brain. Middle: Axial T1 weighted MR image with small punctate white matter lesions. Right: Axial T2 weighted MR image with peri-genuic corpus callosum hyperintense white matter lesion. Discussion Susac syndrome is commonly seen as a clinical triad of encephalopathy, sensorineural hearing loss and visual deficits with supportive imaging. The presented case was noted to have sensorineural hearing loss on audiology examination. However, her inconclusive imaging studies and fluorescein angiography left much to be explained in terms of diagnosis. Discussion Clinically, she met the originally described triad, but we were not able to qualify her symptoms with further testing. It is possible that her peri-genuic corpus callosum lesion represents a new finding for Susac syndrome. The lesion could also have direct corpus callosum involvement that is not fully appreciated on MRI. Other known etiologies had been eliminated. The patient was treated clinically and has improved with immunosuppressive therapy, which further supports her Susac syndrome diagnosis. Discussion Prior to this patient’s diagnosis, years were spent with worsening symptoms and no successful treatment regimen. Factors complicating this syndrome are the difficulty establishing a diagnosis and a non-standardized treatment protocol. Discussion Acute improvement with pulsed steroids and IVIG suggest an autoimmune pathogenesis. One case study report described a post-partum patient whose disease was initially treated with steroids then escalated to cyclophosphamide, aspirin, IVIG, and is now controlled on azathioprine and infliximab.2 To the best of our knowledge, this appears to be the first described clinical case with treatment with anti-tumor necrosis factor alpha agents which further suggests an autoimmune component. Discussion Another case report details a patient with Susac syndrome being treated successfully with rituximab, an anti-CD 20 antibody.5 In addition to our patient’s successful therapeutic regimen, she presented early post-partum which, like other autoimmune diseases such as Rheumatoid Arthritis, further suggests inflammatory pathogenesis. Further studies are ongoing to determine the most appropriate treatment therapies for these patients and to determine symptom pathogenesis. Conclusion Susac syndrome is a complex, likely autoimmune derived disease process. Patients benefit from aggressive and long-term therapies but only after accurate diagnosis which can often take years to establish. To reach a level of early detection and successful treatment regimen for Susac syndrome patients we will require an informed base across multiple disciplines and a coordinated approach to integrated patient care. References 1. Wuerfel, J, Sinnecker, T., Ringelstein, E. “Lesion Morphology at 7 Tesla MRI differentiates Susac Syndrome from Multiple Sclerosis”. Multiple Sclerosis Journal. 18(11) 1592-1599 2012 2. Hardy, T., Garsia, R., Halmagyi G., Lewis S., “Tumor Necrosis Factor (TNF) Inhibitor Therapy in Susac’s Syndrome” Journal of Neurological Sciences. 302(2011) 126-128 3. Lohmann, H. “A Brief Review of Susac Syndrome” Journal of Neurological Sciences. 322(2012) 35-40 4. Magro, C., Poe, J., Lubow, M., Susac, J., “Susac Syndrome - An Organ-Specific Autoimmune Endotheliopathy Syndrome Associated with Anti-Endothelial Cell Antibodies” Am J Clin Pathol 2011; 136:903-912. 5. Lee M, Amezcua I. “Treatment of Susac’s syndrome with Rituximab: a case report.” Neurology 2009; 10:67-74