* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download (eg , heat transfer, energy conversion) in a system.
100% renewable energy wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Kinetic energy wikipedia , lookup
Energy storage wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
World energy consumption wikipedia , lookup
Cogeneration wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Regenerative brake wikipedia , lookup
International Energy Agency wikipedia , lookup
Zero-energy building wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Low-carbon economy wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Alternative energy wikipedia , lookup
Compressed air energy storage wikipedia , lookup
Distributed generation wikipedia , lookup
Energy harvesting wikipedia , lookup
Negawatt power wikipedia , lookup
Gibbs free energy wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Internal energy wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
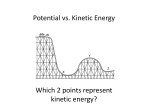
UNDERSTAND PRINCIPLES AND CONCEPTS RELATED TO ENERGY At this level, students should be introduced to energy primarily through energy transformations. Students should trace where energy comes from (and goes next) in examples that involve several different forms of energy along the way: heat, light, motion of objects, chemical, and elastically distorted materials. To change something's speed, to bend or stretch things, to heat or cool them, to push things together or tear them apart all require transfers (and some transformations) of energy. Food, gasoline, and batteries obviously get used up. But the energy they contain does not disappear; it is changed into other forms of energy. Identify forms (e.g. mechanical, chemical) and types (e.g., potential, kinetic) of energy and their characteristics. MEMORIZE 1. Friction is not energy. Friction is a force. 2. Energy is the ability to do work or supply heat. 3. Work is heat and heat is work. Work is the transfer of energy to move an object. 4. Heat is not a form of energy but a method of transferring energy. FIRST LAW OF THERMODYNAMICS – Energy is neither created nor destroyed. Energy is merely transformed or changed from one form to another. SECOND LAW OF THERMODYNAMICS – Heat can’t pass from a colder to a hotter body. And no machine is 100% efficient. Some heat is lost to the environment and some sound is lost. TWO TYPES OF ENERGY - Potential (Position, An object’s energy stored in matter due to position relative to other objects). Kinetic (Moving, the energy of a moving object). A football in the air has both types of energy. KE = ½(m times v times v) or ½ mas times velocity squared. PE = m times g times h. mass of the object times the gravitational pull on the object times the height of the object. Mechanical energy is the total potential plus the total kinetic energy. THE ELEPHANT WITH A “P” ON HIS SWEATER JUMPS OFF A CLIFF. Potential G - Gravitational E - Elastic N – Nuclear (energy in atoms nucleus) C – Chemical (energy in chemical bonds) Kinetic M - Mechanical E – Electric (moving electrons) R – Radiation or EM T – (Thermal or heat) S – Sound. Common Energy Transformations 1. Different types of stoves are used to transform chemical energy of fuel (gas, coal, wood, etc.) into heat. Heat can then make water into steam and turn turbines to make electricity. 2. Solar collectors can be used to transform solar energy into electrical energy. 3. Windmills make use of the kinetic energy of the air molecules, transforming it into mechanical energy that turns turbines to make electrical energy. 4. Hydroelectric plants transform the kinetic energy of falling water into electrical energy. 5. A flashlight converts chemical energy stored in batteries to light energy and heat. Most of the energy is converted into heat, only a small amount is changed into light energy. Demonstrating knowledge of energy transformation and transfers (e.g. , heat transfer, energy conversion) in a system. A slightly more sophisticated proposition is the semi-quantitative one that whenever some energy seems to show up in one place, some will be found to disappear from another. Eventually, the energy idea can become quantitative: If we can keep track of how much energy of each kind increases and decreases, we find that whenever the energy in one place decreases, the energy in other places increases by just the same amount. The energy that is transferred into or out of a system is heat transfer. In a closed system, if one substance loses heat then another substance must gain heat. Heat of fusion is the amount of heat it takes to change from a solid to a liquid or the loss of energy in going from a liquid to a solid. Latent heat – The heat that is required to change a substance from one state to another. Heat of vaporization – the amount of heat that it takes to change from a liquid to a gaseous state. Convection is not so much an independent means of heat transfer as it is an aid to transfer of heat by conduction and radiation. Convection currents appear spontaneously when density differences caused by heating (conduction and radiation) are acted on by a gravitational field. Medium needed How many substances or mediums Travel in Outer space Conduction Yes Convection Yes Radiation No 2 or more No 1 one No None Yes Heat travels through a heated solid or between two heated solids that are touching Heat travels through a fluid (air or liquid) because of changing density. Warm fluids have a larger volume as molecules move faster and farther apart so they are less dense. Heat transfer as the result of electromagnetic waves of traveling photons. The sun warms the earth by emitting radiant energy. Photons are packets of energy. Applying knowledge of the gas laws (e.g. Boyle’s law and Charles’s law). The behavior of gases—such as their compressibility and their expansion with temperature—may be investigated for qualitative explanation; but the mathematics of quantitative gas laws is likely to be more confusing than helpful to most students. PRESSURE is the force exerted on each unit of area of a surface. Pressure is measured in a unit called Pascal. Temperature, Pressure, and Volume are related. Temperature Boyle’s Law Charles’s Law No Change If temperature goes up Pressure If Pressure goes up No Change If temperature goes Then Pressure goes up. A tire on a hot day up because volume or a steel pressure does not change. cooker. Volume Volume goes down. Think of a squeezed balloon. Then volume goes up. Think of the balloon Amber in a hot car. No Change. Thermometers are an example of a one object with a constant volume (thermometer) and one with a changing volume (alcohol or mercury inside). When the temperature goes up, the liquid expands inside because the volume does change. Analyzing phase diagrams (e.g., heat versus temperature) and the flow of energy during changes in states of matter. The horizontal line where a solid object melts and a liquid object freezes is important. On the horizontal line, energy is being added (melting) or lost (freezing) BUT THE TEMPERATURE REMAINS THE SAME because the energy is being used to overcome the intermolecular forces. Heat capacity of an object is the amount of heat energy that it takes to raise the temperature of the object by one degree.