* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Reward and Drug Addiction
Survey
Document related concepts
Polysubstance dependence wikipedia , lookup
Toxicodynamics wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
NMDA receptor wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Norepinephrine wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Neuropharmacology wikipedia , lookup
Transcript
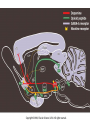
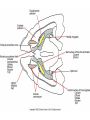
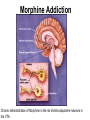
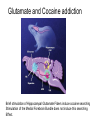
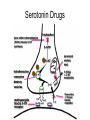
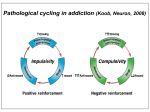
Drugs, Addiction and Reward Stimulants • Behavioral Effect: increase activity, arousal, excitement, etc. • Primary Mechanism of Action: Activation of D2-D4 receptors, either directly or indirectly. • Examples: • Amphetamine: increases release of DA from presynaptic terminal. Increases release of NE. • Cocaine: Blocks reuptake of DA, NE and 5-HT (works on same receptors as Ritalin) • Why the crash? • The cascade following an increase in release (increase DA in the cleft. DA leaves the cleft faster than the presynaptic terminal can resynthesize it. Also, excess DA in the cleft sends feedback via autoreceptors to reduce further release of DA.) More Stimulants • Caffeine: • 1) Vasoconstrictor -- alters blood flow by constricting vessels to the brain, increases blood flow in the short term, but ultimately reduces blood flow to the brain. • 2) Disinhibition -- blocks adenosine, a transmitter that ordinarily inhibits release of glutamate and DA. • Nicotine: • Stimulates nicotinic ACh receptors both in the CNS and in the neuromuscular junction. Can also bind to receptors that release DA in the nucleus accumbens. Depressants • Behavioral Effect: depress CNS -- slower heart rate, reduce tension, reduce anxiety, attentional impairment, memory problems, etc. • Examples: • Alcohol: 1) alters cell membrane properties: inhibits sodium flow across the membrane; expands the membrane surface. 2) Decreases 5-HT activity 3) Makes the GABAa receptor more responsive. (GABA agonist) Hallucinogens • Behavioral Effect: produce a dreamlike state, altered reality, etc. • Examples: • LSD: Stimulates 5-HT receptors. Leads to an increased number of 5-HT receptors at the postsynaptic site. • PCP: Inhibits glutamate receptors (in nuc. accumbens) leading to a net depression of nucleus. • MDMA (Ecstasy): Stimulates release of DA and produces effects at 5-HT synapses. Other Drugs of Abuse: • Marijuana: Active ingredient THC is lipid soluble, binds to cannabinoid receptors in the hippocampus, basal ganglia, and cerebellum. May also alter ACh receptor function. Anandamide: "natural stash“. • Morphine: Opiate receptors; endogenous opiates: enkephalins, endorphins FIGURE 4 Effects of cocaine on thresholds of brain stimulation reward and brain stimulation detection. For measurement of detection threshold, the initial, noncontingent stimulus varied in intensity (at subreward levels), whereas the second, or response-contingent, stimulus was held constant at a rewarding intensity to maintain responding. Each point is the mean z score ± SEM, the difference between the means of the thresholds after administration of vehicle and drug, divided by the standard deviation of all thresholds after vehicle administration. A z score of 2 indicates significant difference from vehicle treatment sessions. These results show that an acute administration of cocaine can lower thresholds of brain stimulation (i.e., facilitate central reward). The cocaine treatment does not affect the ability of the rat to discriminate because detection of a nonrewarding stimulus is not altered (detection threshold). Error bars not shown indicate SEM less than the diameter of the symbols in this illustration. Reprinted with permission from Kornetsky and Bain (1982). FIGURE 2 The cocaine dose–effect function shifts to the right following pretreatment with the dopamine (DA) D-1 receptor antagonists SCH23390 and SCH39166. (A) Effects of pretreatment with SCH23390 (0.01 mg/kg subcutaneous) on the dose–effect function of intravenously selfadministered cocaine (0.06–0.05 mg) measured using the within-session dose–effect paradigm (n=4). (B) Same as in A but for an individual rat. Reprinted with permission from Caine and Koob (1995). (C and D) Effects of pretreatment with SCH39166 on cocaine self-administration in two squirrel monkeys. Points are means based on the last three sessions at each dose of drug. Reprinted with permission from Bergman et al. (1990)., Morphine Addiction Chronic Administration of Morphine in the rat shrinks dopamine neurons in the VTA Alcohol Triggers Opioid Systems Opiate Blockade via Naltrexone administration tends to eliminate “wanting”. Glutamate and Cocaine addiction Brief stimulation of Hippocampal Glutamate Fibers induce cocaine searching Stimulation of the Medial Forebrain Bundle does not induce this searching Effect. Ecstasy Evidence of short-term (B) and longer-term (C) in a monkey after substantive Ecstasy exposure. Serotonergic axon loss is prominent in the anatomy. FIGURE 8 Diagram describing the hypothetical spiraling distress-addiction cycle from a neurobiological perspective. Small arrows refer to increased or decreased functional activity. The addiction cycle is conceptualized as a spiral that increases in amplitude with repeated experience, ultimately resulting in the pathological state of addiction. DA, dopamine; CRF, corticotropin-releasing factor. Reprinted with permission from Koob and Le Moal (1997). Serotonin Drugs