* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Solution
Survey
Document related concepts
Proteolysis wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Restriction enzyme wikipedia , lookup
Western blot wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Deoxyribozyme wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Catalytic triad wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Transcript
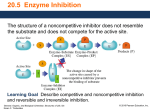
Sample Problem 20.1 The Enzyme Active Site What is the function of the active site in an enzyme? Solution The R groups of amino acid residues within the active site of an enzyme bind the substrate by forming hydrogen bonds, salt bridges, and hydrophobic interactions and catalyze the reaction. Study Check 20.1 Which is a larger molecule, glucose or hexokinase? Answer Hexokinase. Typically, enzymes are large protein molecules containing many amino acid residues, and substrates are small molecules such as the monosaccharide glucose. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.2 The Induced-Fit Model How does the induced-fit model explain the binding of the substrate at the active site? Solution In the induced-fit model, the shapes of the substrate and the active site adjust so that the substrate is in the optimum position needed for the enzyme to carry out reaction catalysis. Study Check 20.2 How does the lock-and-key model explain the binding of the substrate at an active site? Answer In the lock-and-key model, the substrate fits perfectly into the active site of the enzyme similar to a key fitting into a lock. We know today that most enzymes do not have a rigid active site and that the active site flexes to accommodate the chemical reaction being catalyzed. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.3 Classifying Enzymes Using Table 20.2, identify the chemical reaction each of the following enzymes catalyzes and classify the enzyme: a. aminotransferase b. lactate dehydrogenase General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.3 Classifying Enzymes Continued Solution a. An aminotransferase catalyzes the transfer of an amino group between two substrates, which is classified as a transferase. b. Lactate dehydrogenase catalyzes the removal of two H atoms from lactate, which is classified as an oxidoreductase. Study Check 20.3 What is the name and classification of the enzyme that catalyzes the hydrolysis of lipids? Answer An enzyme that hydrolyzes a lipid is a lipase, which is classified as a hydrolase. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.4 Factors Affecting Enzymatic Activity Describe the effect each of the following changes would have on the rate of the reaction that is catalyzed by urease: a. increasing the urea concentration b. lowering the temperature to 10 °C Solution a. An increase in urea concentration will increase the rate of reaction until all the enzyme molecules bind to urea. Then no further increase in rate occurs. b. Because 10 °C is lower than the optimum temperature of 37 °C, there is a decrease in the rate of the reaction. Study Check 20.4 If urease has an optimum pH of 7.0, what is the effect of lowering the pH of a urease sample to 3.0? Answer At a pH lower than the optimum pH, the hydrogen bonds and salt bridges of urease will be disrupted, resulting in the denaturation of the enzyme, loss of tertiary and quaternary structure, and a decrease in its biological activity. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.5 Enzyme Regulation How is the rate of a reaction sequence regulated in feedback control? Solution When the end product of a reaction sequence is produced at sufficient levels for the cell, some product molecules bind to the allosteric enzyme (E1) in the sequence, which shuts down all the reactions that follow and stops the synthesis of intermediate products. Study Check 20.5 Why is pepsin, a digestive enzyme, produced as a zymogen? Answer Pepsin hydrolyzes proteins in the foods we ingest. It is synthesized as a zymogen, pepsinogen, to prevent its digestion of the proteins that make up the organs in the body. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.6 Enzyme Inhibition State the type of inhibition in the following: a. The inhibitor has a structure similar to that of the substrate. b. The inhibitor binds to the surface of the enzyme, changing its shape in such a way that it cannot bind to substrate. Solution a. Step 1 Compare the structure of the inhibitor to that of the substrate. This inhibitor has a structure similar to that of the substrate. Step 2 Describe the characteristics of the inhibitor. This inhibitor would compete with the substrate for the active site. Step 3 Assign the type of inhibition based on the characteristics. This type of inhibition is competitive inhibition, which is reversed by increasing the concentration of the substrate. b. Step 1 Compare the structure of the inhibitor to that of the substrate. This inhibitor does not have a structure similar to the substrate. Step 2 Describe the characteristics of the inhibitor. This inhibitor binds to the surface of the enzyme where it changes the shape of the enzyme and the active site. Step 3 Assign the type of inhibition based on the characteristics. This type of inhibition is noncompetitive inhibition. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.6 Enzyme Inhibition Continued Study Check 20.6 What type of inhibition occurs when aspirin, also called acetylsalicylic acid, forms a covalent bond with serine in the active site of the enzyme cyclooxygenase? Answer irreversible inhibition General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.7 Cofactors Indicate whether each of the following enzymes is active with or without a cofactor: a. an enzyme that needs Mg2+ for catalytic activity b. a polypeptide chain that is biologically active c. an enzyme that binds to vitamin B6 to become active Solution a. The enzyme would be active with the metal ion Mg 2+ cofactor. b. An active enzyme that is only a polypeptide chain does not require a cofactor. c. An enzyme that requires vitamin B6 is active with a cofactor. Study Check 20.7 Which cofactor for the enzymes in Sample Problem 20.7 would be called a coenzyme? Answer vitamin B6 General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc. Sample Problem 20.8 Vitamins Why is it more important to get regular, daily amounts of vitamins B 1 and B2, but not vitamins A or D? Solution Water-soluble vitamins like vitamin B1 (thiamine) and vitamin B2 (riboflavin) are not stored in the body, whereas fat-soluble vitamins such as vitamin A (retinol) and vitamin D (cholecalciferol) are stored in the liver and body fat. Any excess of thiamine or riboflavin is eliminated in the urine and must be replenished each day from the diet. Study Check 20.8 Why are fresh fruits rather than cooked fruits recommended as a source of vitamin C? Answer Water-soluble vitamins such as vitamin C are easily destroyed by heat. General, Organic, and Biological Chemistry: Structures of Life, 5/e Karen C. Timberlake © 2016 Pearson Education, Inc.