* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
Fatty acid synthesis wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Photosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Metalloprotein wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
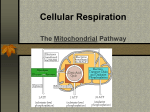
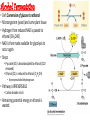
Cell Respiration Part 5 Anaerobic Respiration, Respiratory Quotient, and Rice Adaptations Anaerobic Respiration • No free oxygen = nothing pulling electrons and hydrogen ions into the mitochondria • Remember, reduced NAD from glycolysis are supposed to head into mitochondria for ETC • No oxygen = no mitochondria for those electron carriers • ETC stops working • No large supply of ATP from oxidative phosphorylation • 1 glucose 4 ATP (2 net ATP) and 2 reduced NAD • 2 anaerobic pathways solve problem of “dumping” hydrogen from reduced NAD • Lactic Fermentation • Alcohol Fermentation Anaerobic Respiration • “Fermentation” • Lactic Fermentation and Alcohol Fermentation • Both: • • • • • Occurs in absence of oxygen Take place in Cytoplasm Begin with glycolysis Regenerate NAD Enable glycolysis to occur again to produce more ATP • “Buy time” by making small amounts of ATP • Both products (lactate and ethanol) are toxic to organism • Reactions cannot continue indefinitely Alcohol Fermentation • Def: Conversion of glucose to ethanol • Microorganism (yeast) and some plant tissue • Hydrogen from reduced NAD is passed to ethanal (CH3CHO) • NAD is then made available for glycolysis to occur again • Steps: • Pyruvate (3C) is decarboxylated to ethanal (CO2 removed) • Ethanal (2C) is reduced to ethanol (C2H5OH) • By enzyme alcohol dehydrogenase • Pathway is IRREVERSIBLE • Carbon dioxide is lost • Remaining potential energy in ethanol is wasted Lactic Fermentation • Def: Conversion of glucose to lactic acid • Microorganisms and mammalian tissue • Pyruvate (3C) acts as a hydrogen acceptor • Reduced NAD drops of hydrogen to pyruvate, yielding a lactate molecule (3C) • By enzyme Lactate dehydrogenase • Named after reverse reaction (which it also catalyzes) • NAD is then made available for glycolysis to occur again in anaerobic conditions • Pathway REVERSIBLE Lactic Fermentation • Pathway REVERSIBLE • Lactate carried by blood plasma to liver • Lactate converted back to pyruvate in liver • Liver oxidizes 20% of incoming lactate to carbon dioxide and water via aerobic respiration (when oxygen is available again) • 80% remaining lactate is converted by liver into glycogen Lactic Fermentation • Figure to the left shows what happens to oxygen uptake of a person before, during, and after strenuous exercise • At Rest = absorbing oxygen at less than 1000 mL min-1 • Beginning exercise = more oxygen needed to support aerobic respiration in muscles A. B. C. D. E. Demand for oxygen begins increasing Takes heart & lungs 2 minutes to meet demand Lactic acid fermentation occurs in the mean time Person builds up oxygen deficit Next few minutes, enough oxygen is supplied • Exercise ends = person breathing deeply, absorbing oxygen at higher rate than at rest • Extra oxygen uptake after work out is to pay that oxygen deficit established at the beginning of working out • Oxygen debt post-exercise uptake of extra oxygen • Oxygen needed for: • Conversion of lactate to glycogen in liver • Reoxygenation of hemoglobin in blood • A high metabolic rate E Respiratory Substrates • Cells require substrates to breakdown for energy • Glucose not the only substrate used for energy • Neurons, RBCs, and lymphocytes can oxidize glucose • Brain neurons can ONLY respire Glucose • Other cells can oxidize lipids and amino acids • Lipids as a substrate • Carbon atoms removed in pairs (acetyl coenzyme A) from fatty acid chains of triglycerides • These pairs enter Krebs cycle • Amino Acids as a substrate • Carbon-hydrogen skeleton converted into pyruvate or acetyl coenzyme A Energy Values of Respiratory Substrates • Where does most energy come from in aerobic respiration? • Oxidation of hydrogen to water when reduced NAD and reduced FAD get to the ETC • Main Idea: • The greater the number of the hydrogens in the substrate molecule = the greater the energy value • “Energy Density” energy value per unit mass (aka amount of energy packed into a substrate molecule) • Fatty acids have more hydrogens than carbohydrates • Therefore, fatty acids have greater energy density Measuring Energy of Substrates • How to determine energy in s substance • Burn know mass of substance in a calorimeter • Calorimeter measures the energy value of a respiratory substrate • Energy released by burning (oxidizing) substrate will cause the a known mass of water’s temperature to rise • Measuring the change in water temperature will determine energy value of substrate oxidized Respiratory Substrates Respiratory Substrate Energy Released/kJ Carbohydrate 16 Lipid 39 protein 17 Respiratory Substrate RQ Carbohydrate 1.0 Lipid 0.7 protein 0.9 g-1 • Lipids provide TWICE as much energy per gram as carbs or proteins • Lipids contain more carbonhydrogen bonds (more H atoms) • H atoms used to generate the most ATP is oxidative phosphorylation • Brain cells only use glucose • Heart muscle prefers fatty acids • Other cells carbs, lipids, or fats Determining Respiratory Quotient • Two ways: • Look at Carbon Dioxide produced • Look at Oxygen used • Use: • Volume (if given volume/data table/graph) • Moles (if given equation) Determining Respiratory Quotient Anaerobic • Alcohol Fermentation: • • • • No oxygen is used… Dividing carbon dioxide produce by 0 RQ = infinity Yeast use some aerobic respiration so small amounts of oxygen are used…. • High values of RQ indicate alcohol fermentation is occurring • Lactic Fermentation • No oxygen is used… • No carbon dioxide produced…. • No respiratory quotient can be determined Practice problems 1. During 30 minutes of steady-state exercise a subject averages an oxygen consumption of 3.22 L/min with a CO2 production of 2.78 L/min. Determine RQ 2. Determine respiratory quotient of complete oxidation of oleic acid (C18H34O2) from olive oil. Hint: balance equation first….oxygen can be half a molecule. Answers 1. RQ or RER = 2.78 / 3.22 = 0.86 2. RQ= 18/25.5= 0.7 (C18H34O2) + 25.5O2 18 CO2 + 17H2O + energy

















![fermentation[1].](http://s1.studyres.com/store/data/008290469_1-3a25eae6a4ca657233c4e21cf2e1a1bb-150x150.png)