* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biofilm and Chronic Infections
Infection control wikipedia , lookup
Gastroenteritis wikipedia , lookup
Community fingerprinting wikipedia , lookup
Metagenomics wikipedia , lookup
Phospholipid-derived fatty acids wikipedia , lookup
Urinary tract infection wikipedia , lookup
Antimicrobial surface wikipedia , lookup
Neonatal infection wikipedia , lookup
Quorum sensing wikipedia , lookup
Anaerobic infection wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Marine microorganism wikipedia , lookup
Antibiotics wikipedia , lookup
Staphylococcus aureus wikipedia , lookup
Disinfectant wikipedia , lookup
Magnetotactic bacteria wikipedia , lookup
Probiotics in children wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Human microbiota wikipedia , lookup
Triclocarban wikipedia , lookup
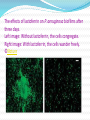
Autism ONE Chicago May 2009 Anju Usman,MD True Health Medical Center Many patients with autistic symptoms have persistent dysbiosis. Treatment of GI issues often alleviates the symptoms we call autism. Hypothesis: Patients with autism, who have toxic metal burdens and toxic chemical burdens are likely to grow resistant organisms in their GI tract. This resistance to treatment is perpetuated by the production of biofilms. Treatment of biofilms will help to eradicate dysbiotic flora and improve the symptoms we call autism. BIOFILM What is Biofilm? What is the implication of biofilm production in ASD? How are they formed? Where do they grow? Possible treatments being researched? What is Biofilm? A biofilm is a collection of microbial communities enclosed by a matrix of extracellular polymeric substance (EPS) and separated by a network of open water channels. These communities adhere to manmade and natural surfaces, such as metals and teeth, typically at a liquid-solid interface . Their architecture is an optimal environment for cell-cell interactions, including the intercellular exchange of genetic material, communication signals, and metabolites, which enables diffusion of necessary nutrients to the biofilm community. The matrix is composed of a negatively charged polysaccharide substance, held together with positively charged metal ions (calcium, magnesium, and iron). Staphylococcus aureus biofilm The matrix in which microbes in a biofilm are embedded protects them from UV exposure, metal toxicity, acid exposure, dehydration salinity, phagocytosis, antibiotics, antimicrobial agents and the immune system. How is Biofilm formed? 5 stages of biofilm development. Stage 1, initial attachment; stage 2, irreversible attachment; stage 3, maturation I; stage 4, maturation II; stage 5, dispersion. Each stage of development in the diagram is paired with a photomicrograph of a developing P. aeruginosa biofilm. Nature: May 2002 Where do they grow? •Biofilm formation appears common near polluted and toxic areas and environments. Account for the majority of all microbial infections of the human body. • Device-related infections, intravenous catheters, joint prostheses • •Human body – Mouth, teeth, pancreaticbiliary tracts, lungs, sinuses, adenoids, tonsils and the intestinal tract…. Why are they so difficult to treat? • Remarkably difficult to treat with antimicrobials, resistant to doses of antimicrobials 100- to 1000-fold over the minimum lethal dose for microbes outside of biofilms. • Antibiotics do not penetrate the polysaccharide matrix. • Highly resistant to both immunological and non-specific defense mechanisms of the body. • Difficult to diagnose, difficult to culture. • Microbes impart genetic material to one another to maintain resistance. • Colonies communicate with one another through the use of quorum sensing molecules. • Colonies fail to express OMP (outer membrane proteins). “Testing the Susceptibility of Bacteria in Biofilms to Antibacterial Agents” Antimicrobial Agents and Chemo. Nov 1990. Quorum Sensing bacteria communicate with each other via signal molecules or autoinducers Autoinducers drive bacterial gene expression and regulate Bioluminescence Virulence Biofilm Conjugation Motility Presence of Normal Bacteria QS signals can be blocked Gram negative bacteria produce lactones E. coli produce epinephrine Brominated furanones (marine algae) are quorum sensing inhibitors Gram positive bacteria produce cyclic peptides RIP, a synthetic peptide, interferes with biofilm infections in animal models “Cell to Cell Signaling in Intestinal Pathogens” Curr Issues Intest Microbiol,Mar 2004 “Bacteria-Host Communication: The Language of Hormones”Microbiology. May 2003 Is it possible my child has Biofilm producing bacteria and yeast overgrowth? Consider if Persistent /Recurrent Dysbiosis Recurrent Sinusitis/Otitis Initially does well with Antibiotics and Antifungals History of frequent Antibiotics Frequent flaring of yeast/bacteria during DETOX Concomitant – gingivitis, tonsillitis, dental caries Stool and Urine Cultures negative, but patient does well when placed on antifungals/antibiotics What type of biofilm control strategies are being researched? • Probiotics and Prebiotics • EDTA • Iron chelating compounds • Enzymes - mucous degrading • Others Probiotics to Prevent the Need For, and Augment the Use Of, Antibiotics Can J Infect Dis Med Microbiol. 2006 Sep Gregor Reid, BSc, PhD MBA Reduce the risk of antibiotic-induced super infections in the gut and the vagina; Secrete antibacterial substances that lower pathogenic bacterial populations locally and at distant mucosal sites, and disrupt biofilms, making it easier for antibiotics to function; Enhance generalized mucosal immunity, which in turn aids in the eradication of the organisms at the mucosal site. Microbial biofilms in the human gastrointestinal tract. J Appl Microbiol. 2007 May;102(5):1187-96. Macfarlane S, Dillon JF. There is mounting interest in mucosal biofilms in the colon, especially with respect to their role in inflammatory bowel disease. Because bacteria growing in biofilms are more resistant to antibiotics than unattached organisms, it is often difficult to modify the structure and composition of these communities, or to eradicate them from the body. However, recent work has shown that there is considerable potential to alter the species composition of mucosal biofilms in a beneficial way using synbiotics. The Efficacy of EDTA Against Biofilm Bacteria (Kim, 2005) Biofilms = complex communities of micro-organisms attached to surfaces held together by EPS (extracellular polysaccharides, that are negatively charged and held together by positively charged cations, specifically Fe2+, Ca2+, and Mg2+. EDTA complexes with cations in the extracellular matrix. Neither Vancomycin or EDTA alone detached Staph biofilm. EDTA plus Vancomycin together caused biomass removal. Chelator-Induced Dispersal and Killing of Pseudomonas aeruginosa Cells in a Biofilm (Banin, 2005) EDTA is a potent Pseudomonas biofilm disrupter. 1000x killing when EDTA combined with Gentamicin. EDTA causes dispersal and killing of biofilm cells. Ca, Fe, and Mg protect biofilm. When Ca or Fe are added, killing and detachment are completely blocked. EDTA as an Adjuct Antifungal Agent for Invasive Pulmonary Aspergillosis in a Rodent Model (Hachem, 2006) Immunosuppressed rats infected with Aspergillus who were treated with an antifungal drug and EDTA in combination had less severe disease and greater survival EDTA acts like an antifungal –enhancing agent Absence of direct toxic effect of EDTA, no tissue damage associated with EDTA Iron Chelating Compounds Outer membrane proteins(OMP) are expressed when iron is restricted. If OMP are not expressed, the immune system is not alerted appropriately, and can not illicit a normal immune response. Transferrin and Lactoferrin Synthesized by host to inhibit bacterial growth by sequestering free Iron. Pathogenic bacteria secrete iron chelators (siderophores) to compete with transferrin and lactoferrin for Iron. A Component of Innate Immunity Prevents Bacterial Biofilm Development. Nature. 2002 May 30;417(6888):552-5. Singh PK, Parsek MR, Greenberg EP, Welsh MJ. Antimicrobial factors form one arm of the innate immune system, which protects mucosal surfaces from bacterial infection. These factors can rapidly kill bacteria deposited on mucosal surfaces and prevent acute invasive infections. In many chronic infections, however, bacteria live in biofilms, which are distinct, matrix-encased communities specialized for surface persistence. The transition from a freeliving, independent existence to a biofilm lifestyle can be devastating, because biofilms notoriously resist killing by host defence mechanisms and antibiotics. We hypothesized that the innate immune system possesses specific activity to protect against biofilm infections. Here we show that lactoferrin, a ubiquitous and abundant constituent of human external secretions, blocks biofilm development by the opportunistic pathogen Pseudomonas aeruginosa. This occurs at lactoferrin concentrations below those that kill or prevent growth . By chelating iron, lactoferrin stimulates twitching, a specialized form of surface motility, causing the bacteria to wander across the surface instead of forming cell clusters and biofilms. These findings reveal a specific anti-biofilm defence mechanism acting at a critical juncture in biofilm development, the time bacteria stop roaming as individuals and aggregate into durable communities. The effects of lactoferrin on P. aeruginosa biofilms after three days. Left image: Without lactoferrin, the cells congregate. Right image: With lactoferrin, the cells wander freely. ©Nature Enzymatic Degradation Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A. 2007 Jul 3;104(27):11197-202. Epub 2007 Jun 25. Lu TK, Collins JJ. Synthetic biology involves the engineering of biological organisms by using modular and generalizable designs with the ultimate goal of developing useful solutions to real-world problems. One such problem involves bacterial biofilms, which are crucial in the pathogenesis of many clinically important infections and are difficult to eradicate because they exhibit resistance to antimicrobial treatments and removal by we engineered bacteriophage to express a biofilm-degrading enzyme during infection to simultaneously attack the bacterial cells in the biofilm and the biofilm matrix, which is composed of extracellular polymeric substances. We show that the efficacy of biofilm removal by this twopronged enzymatic bacteriophage strategy is significantly greater than that of nonenzymatic bacteriophage treatment. Our engineered enzymatic phage substantially host immune systems. To address this issue, reduced bacterial biofilm cell counts by approximately 4.5 orders of magnitude ( approximately 99.997% removal), which was about two orders of magnitude better than that of nonenzymatic phage. This work demonstrates the feasibility and benefits of using engineered enzymatic bacteriophage to reduce bacterial biofilms and the applicability of synthetic biology to an important medical and industrial problem. Biofilm formation of Staphylococcus aureus strains isolated from impetigo and furuncle: role of fibrinogen and fibrin. J Dermatol Sci. 1997 Nov Akiyama H, Ueda M, Kanzaki H, Tada J, Arata J. The formation of membranous structure (thickness from the plastic tissue-culture coverslip (hematoxylineosin) > 1 mm; periodic acid-Schiff-positive) was more prominent with Staphylococcus aureus (S. aureus) strains isolated from impetigo (coagulase types I.V origin) than with S. aureus strains isolated from furuncle (coagulase type IV origin) (P < 0.05) in the plastic tissue-culture coverslip in human plasma after 72 h. Attachment of S. aureus cells to a plastic tissue-culture coverslip was more marked in 0-3% fibrinogen/tryptic soy broth (TSB) than in plasma (P < 0.05). The formation of the membranous structure was observed on the plastic tissue-culture coverslip with 0.3% fibrinogen/human serum but not with 0.3% fibrinogen + 5% glucose/TSB. Electron microscopy revealed abundant fibrin around S. aureus cells at 4 h and Ruthenium red-positive materials increased at 24 and 72 h in plasma. Staphylococcus aureus cell attachment to the plastic tissue-culture coverslip in plasma decreased by addition of levofloxacin (LVFX) at 1/2 minimum inhibitory concentration (MIC) and clarithromycin (CAM) at 1/4 MIC. Polysaccharide production of S. aureus cells on the plastic tissueculture coverslip in plasma decreased with the addition of CAM at 1/4 MIC. Fibrinogen is closely related to initiation of infection but biofilm formation requires the conversion of fibrinogen to fibrin. Thus, attachment of S. aureus cells to the plastic tissue-culture coverslip, conversion of fibrinogen to fibrin by coagulase-prothrombin complex, and production of abundant glycocalyx by S. aureus cells are at least required for the production of biofilm in staphylococcal skin infection. Proteolytic enzymes: a new treatment strategy for prosthetic infections? Antimicrob Agents Chemother. 1993 Dec Selan L, Berlutti F, Passariello C, Comodi-Ballanti MR, Thaller MC. Among the different mechanisms of bacterial resistance to antimicrobial agents that have been studied, biofilm formation is one of the most widespread. This mechanism is frequently the cause of failure in the treatment of prosthetic device infections, and several attempts have been made to develop molecules and protocols that are able to inhibit biofilm-embedded bacteria. We present data suggesting the possibility that proteolytic enzymes could significantly enhance the activities of antibiotics against biofilms. Antibiotic susceptibility tests on both planktonic and sessile cultures, studies on the dynamics of colonization of 10 biofilm-forming isolates, and then bioluminescence and scanning electron microscopy under seven different experimental conditions showed that serratiopeptidase greatly enhances the activity of ofloxacin on sessile cultures and can inhibit biofilm formation. Enhancement of the Fibrinolytic Activity in Plasma by Oral Administration of Nattokinase. Acta Haematol. 1990;84(3):139-43 Sumi H, Hamada H, Nakanishi K, Hiratani H. The existence of a potent fibrinolytic enzyme (nattokinase, NK) in the traditional fermented food called 'natto', was reported by us previously. It was confirmed that oral administration of NK (or natto) produced a mild and frequent enhancement of the fibrinolytic activity in the plasma, as indicated by the fibrinolytic parameters, and the production of tissue plasminogen activator. NK capsules were also administered orally to dogs with experimentally induced thrombosis, and lysis of the thrombi was observed by angiography. The results obtained suggest that NK represents a possible drug for use not only in the treatment of embolism but also in the prevention of the disease, since NK has a proven safety and can be mass produced. Dentists understand biofilm! “Normal mouthwashes can only clean the surface, which is why bad breath returns quickly and gum disease is a constant problem. With the new patented technology in Biotene PBF mouthwash, you can dissolve the biofilm, expose hidden bacteria colonies and kill germs. In addition, Biotene PBF contains the proven LP3 salivary enzyme system to strengthen the body’s antibacterial action, dissolving biofilm and inhibiting excessive bacteria – maintaining a healthy oral balance.” Chitosans and Biofilm Effects of chitosans with different molecular weights on Streptococcus sanguis biofilm Ma R, Zhu M, Liu Z. Streptococcus sanguis biofilm was formed on saliva-coated glass (SCG) in a flow culture system, then exposed to 2% chitosans with different molecular weights (5 cps, 80 cps, 600 cps) for 3, 10, 30 minutes. Confocal laser scanning microscope and Vital/Dead fluorescent staining technique (vital stained green, dead stained red) were combined to observe the biofilm thickness, bacterial density. Analysis of variance was used for PMID: the biofilm thickness and bacterial density reduced significantly after treatment with 2% chitosan. Low molecular weight chitosan seems most effective at detaching biofilms. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. RESULTS: Food Microbiol. 2008 Oct .Fernandes JC True Health Medical Center Gut Biofilm Approach Step 1 Lysis/Detachment Step 2 Microbial Killing Step 3 Clean up Step 4 Rebuilding/Nourishing the Gut Lining Step 1: Lysis/Detachment Enzyme (polysaccharidase, disaccharidase) Staph use fibrinolytic like lumbrokinase, serratiopeptidase, or nattokinase Pseudomonas or thick mucus production use serratiopeptidase May use pineapple or papaya for sensitive patient Disodium EDTA (oral only) or Apple Cider Vinegar Lactoferrin (especially for Pseudomonas) NAG/Chitosan (especially for Strep Biofilm, do not give if shellfish allergy) Works best on an empty stomach Start one supplement at a time, go slow Do not give Enzymes on an empty stomach to patients with severe GI issues Do not give Lactoferrin to patients with dairy allergy Avoid giving Iron, Calcium or Magnesium with supplements above Careful with sensitive patients or weak patients Step 2: Killing Consider Natural Antimicrobials first Do not start with Pharmaceuticals Vary agents depending on microbiology and mycology testing Bacterial Cultures tend to show Gram positive overgrowth or Gram negative Dysbiosis may seem to worsen initially Watch for die off, treat accordingly Consider the use of antibiotics or antifungal meds if organisms remain persistent Start low and go slow Step 3: Clean up Fiber, insoluble/soluble Activated Charcoal if needed Alginates, Brown Algae Modified Citrus Pectin Very important step Helps prevent symptoms of die off Step 4: Rebuilding/ Nourishing the Gut Lining Probiotics Prebiotics Fermented Foods Healing, nutritious, non-toxic foods Supportive Nutrients Microbial Flora Bacteria are a major component of colonic material Hundreds of species/strains exist in the intestines Metabolic activity affects the host Digestion, Energy Production, Metabolism Modulation of the immune system Destruction of toxins and mutagens Repression of pathogenic microbial growth Preventing allergy Preventing inflammatory bowel disease and inflammation The Colon has an obligate need for bacterial fermentation products (SCFA, short chain fatty acids) “the species composition and biochemical activities of the microbial flora are determined primarily by diet and are strongly influenced by carbohydrate availability” “Composition and Metabolic Activities of Bacterial Biofilms Colonizing Food Residues in the Human Gut” (Macfarlane Sept 2006) Probiotics, Prebiotics, and Synbiotics: Approaches for Modulating the Microbial Ecology of the Gut M David Collins and Glenn R Gibson American Journal of Clinical Nutrition, May 1999 Probiotics A Lactobacillus acidophilus supplement given to formula-fed infants was thought to improve weight gain. Oral rehydration that included a strain of L. casei promoted recovery from acute diarrhea in children. Oral administration of L. acidophilus has also been shown to be effective against bacterially induced gastroenteritis . Applications of bifidobacterial probiotics in infants have been directed toward reducing the growth of Candida albicans and the incidence of enterocolitis. Other organisms such as E. coli and the yeast. Saccharomyces boulardii have been reported to have some beneficial effects in maintaining remission in IBD (Kruis, Rembacken, Guslandi ). Prebiotics are nondigestible food ingredients that beneficially affects the host by selectively stimulating the growth of beneficial bacteria(inulin, oligosaccharides) Synbiotics are a combination of both prebiotics and probiotics Probiotics – beneficial bacteria Bifidobacterium (breve, longum, infantis) Lactobacillus (acidophilus, casei, paracasei, johnsonii, lactis, plantarum, reuteri, rhamanosus, salivarius ) Escherichia coli Enterococcus Streptococcus thermophilus D- Lactic Acid Free Anti-oxidant rich foods help reduce inflammation Inadequate antioxidant status is a major pathway for inflammation. Various free rdicals (ROS), including superoxide, peroxide, hydroxyl and peroxynitrite, are generated through the inflammatory prostaglandin/leukotriene pathways. These free radicals can damage or destroy virtually every cellular biomolecule: proteins, fatty acids, phospholipids, glycoproteins, even DNA, leading to cell injury or death. vitamins C and E are the two most important nutritional antioxidants. Vitamin C, E, alpha-lipoic acid, Co Q10 and NADH act as a team. One of the many ways pro-oxidants damage neurons is to prevent the intracellular formation of glutathione. Biofilm Reducing Diet Strategies Organic is best, Keep toxin exposure low Hormone-free, antibiotic-free, pesticide free, grass-fed meat Consider Specific Carbohydrate Diet (SCD) Use Digestive Enzymes that break down Carbohydrates Choose foods with high ORAC values ORAC corresponds to the amount of antioxidants in foods Fresh Vegetables and Fruits are rich in antioxidants (juicing) Choose foods with low AGEs(advanced glycation endproducts) Exogenous glycations and AGEs are typically formed when sugars are cooked with proteins or fats at high temperatures High exogenous AGEs includes: donuts, barbecued meats, cake, dark colored soda pop, and french fries Start fermenting foods Add kombucha , kefir and cultured foods Supportive Healing Nutrients Xylitol, Aloe, Ribose, Omega 3 Essential Fatty Acids Okra, Slippery Elm, Marshmellow Root, Ginger N-Acetyl Glucosamine Supports gastrointestinal function by enhancing mucosal integrity Provides a key building block molecule for connective tissue formation Better tolerated than glucosamine sulfate for those sensitive to sulfur Ecklonia cava rich in minerals, trace elements, iodine and especially mucopolysaccharides. Fucoidans, component of brown seaweeds, a group of sulfated polysaccharides powerful anti-oxidant properties protect cellular DNA from free radical damage Laminaran, a complex sugar with laxative and diuretic properties. Colostrum / Whey Insulin-like growth factors (IgF) which stimulates cellular growth and repair, Transforming Growth Factors (TgF) which promote the synthesis and repair of RNA and DNA and the repair of muscle tissue (particularly helpful for athletes), Epithelial Growth Factor EfG (which stimulates normal skin growth). Potential Reactions Symptoms Irritability, aggression, behavioral issues Increased stimming, hyperactivity, sleeplessness Skin rash, diaper rash, fever Possible Causes Side effect of supplement or allergy to med Yeast or Bacterial Flare-up (Balancing act) Detox Reaction = Too rapid of an exodus of heavy metals leading to vitamin or mineral deficiency, oxidative stress, liver or kidney stress Die off = Rapid death of gut bugs, leading to excess release of toxins such as ammonia and subsequent liver or kidney stress Immune/Inflammatory??? = reaction to gut bugs Our Statistics and Case Discussions Biofilm Questionnaire n=20 Parent Comments and Observations Labs Biofilm Questionnaire 20 Random Patients Average duration of protocol 4.6 months (1 – 12 mo) Parents rated benefit Overall benefit (0 – 10) 16/20 reported positive changes 4/20 reported no or little change 2 – quit early due to negative behaviors, which resolved after stopping protocol 2 – no negatives, no positives 1 – no changes in ASD symptoms, positive effect on Hg detox Patient Reported Benefits of Gut-Biofilm Protocol n = 20 10 9 8 7 6 # of Patients 5 4 3 2 1 0 Not helpful Mildly helpful Moderately helpful Very helpful Parent Comments and Observations “the biofilm protocol was able to accomplish in one year what 5 6 yrs of GI meds were not” “ASO titers improved after years of unsuccessful treatments” “couldn’t sit still, stimming worsened, poor attention, OCD increased while on protocol” “improved spontaneous conversation, open to new foods” “better appetite, healthier, gained weight for first time” “initial regression followed by improved cognitive, reading, language, processing, and conversation” “language doubled, more aware, more defiant” “decreased OCD and motor tics dramatically” “developed a strep infection during the protocol” “improved verbal scripting, less tantrums, improved social play, better school work” Take home pearls Drink clean, non-toxic water Eat healthy, antioxidant rich, non-toxic food Let go of negative toxic thoughts Get plenty of pure sunshine Remember to laugh Remember to play Take care of yourself and your gut as well “If you heal the gut, you heal the brain” Jane Casey Author and mother of twins My Kids Won’t Eat it!! Neither would mine You can do this, I did with twins!!! A thoughtful approach…. What Diet should I implement? Gluten-Free/Casein-Free?? Gradually start taking things out of the diet. And try not freak out. After 3-6 month trial consider re-introduction Unless your child has a severe allergy, start adding healthy, organic, fermented dairy or whey products. Start with dairy fat not protein, like ghee, butter, cream, sour cream Consider sprouted organic grains What is working for me? Alkalizing Drink upon first waking i.e. lemon water, etc. Probiotics Kombucha Coconut Oil Raw Butter Mixed with a little Manuka honey ¾ tsp raw butter with ½ tsp honey Thank You!! PHONE: (630) 995-4242 FAX: (630) 995-4243