* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download File - LSAmockscience
Chemical weapon proliferation wikipedia , lookup
Chemical weapon wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical element wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemical bond wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical industry wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Safety data sheet wikipedia , lookup
Drug discovery wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemical potential wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Marcus theory wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Process chemistry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Click chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
History of molecular theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Chemical reaction wikipedia , lookup
Transition state theory wikipedia , lookup
Atomic theory wikipedia , lookup
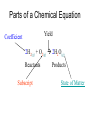
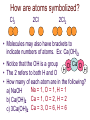
Chemical Reactions and Equations Chapter 7 • http://www.youtube.com/watch?feature=pl ayer_embedded&v=dUMmoPdwBy4 What is a Chemical Reaction? • One or more substances are converted to one or more new substances Why do Chemical Reaction Occur? • Chemical stability • Atoms attempt to obtain a full set of valence electrons (octet rule) Parts of a Chemical Reaction • Reactant(s) – Starting substance(s) – Left side of equation • Product(s) – Substance(s) produced – Right side of equation – Example 2Mg + Reactants O2 2MgO Yield Product What are Chemical Equations? • Representations of chemical reactions as words or formulas • 2 Types of chemical equations – Word Equations – use the chemical names in the equation – Formula Equations – use the formulas in the equation Law of Conservation of Matter In a chemical reaction: The mass of Reactants always = the mass of the Products. Law of Conservation of mass = mass is neither created or destroyed in a chemical reaction. Why balance chemical equations • Law of Conservation of Mass – Mass can not be created or destroyed • In a balanced chemical equation – The number of atoms of each element on the reactant side must equal the number of atoms of each element on the product side Parts of a Chemical Equation Yield Coefficient 2H2(g) + O2(g) 2H2O(g) Reactants Subscript Products State of Matter How are atoms symbolized? Cl2 2Cl 2Cl2 • Molecules may also have brackets to indicate numbers of atoms. Ex: Ca(OH)2 • Notice that the OH is a group O Ca O H • The 2 refers to both H and O H • How many of each atom are in the following? Na = 1, O = 1, H = 1 a) NaOH b) Ca(OH)2 Ca = 1, O = 2, H = 2 c) 3Ca(OH)2 Ca = 3, O = 6, H = 6 Balancing equations: • The law of conservation of mass states that mass can neither be created or destroyed • So, atoms are neither created or destroyed, only rearranged in a chemical reaction • So, the number of a particular atom is the same on both sides of a chemical equation • Example: Magnesium + Oxygen • Mg + O2 MgO Mg + O O Mg O • However, this is not balanced • Left: Mg = 1, O = 2 • Right: Mg = 1, O = 1 To Balance Equations: 1. Count the number of atoms of each element on both sides of the equation. 2. Place coefficients in front of the proper elements or compounds in order to get the same number of atoms on both sides of the equation. 3. Make sure the coefficients are in the lowest whole-number ratio. 4. Check your work by counting the atoms of each element. Mg + O2 MgO becomes _____________________ Examples: Mg + HCl MgCl2 + H2 Mg = 1 H=1 Cl = 1 Mg = 1 H=2 Cl = 2 Examples Mg + 2HCl MgCl2 + H2 Mg = 1 H=1=2 Cl = 1 = 2 Mg = 1 H=2 Cl = 2 Balance these equations: a) b) c) d) e) f) g) h) Ca + N2 Ca3N2 NH4NO3 N2O + H2O BiCl3 + H2S Bi2S3 + HCl C4H10 + O2 CO2 + H2O O2 + C6H12O6 CO2 + H2O NO2 + H2O HNO3 + NO Cr2(SO4)3+ NaOH Cr(OH)3+ Na2SO4 Al4C3 + H2O CH4 + Al(OH)3 Balance these equations: a) b) c) d) e) f) g) h) 3Ca + N2 Ca3N2 NH4NO3 N2O + 2H2O 2BiCl3 + 3H2S Bi2S3 + 6HCl 2C4H10 + 13O2 8CO2 + 10H2O 6O2 + C6H12O6 6CO2 + 6H2O 3NO2 + H2O 2HNO3 + NO Cr2(SO4)3+ 6NaOH 2Cr(OH)3+ 3Na2SO4 Al4C3 + 12H2O 3CH4 + 4Al(OH)3 Writing Formula Equations from Word Equations Things to remember: There are elements that naturally form diatomic molecules and come in pairs: Br2 I2 N2 Cl2 H2 O2 F2 You must write the correct formula for the word formula!!!!! Write the formula equation for the reaction in which magnesium reacts with nitrogen to produce magnesium nitride 1. Write the word equation: magnesium + nitrogen magnesium nitride 2. Determine if any of the elements are diatomic: H2, O2, N2, Cl2, Br2, I2, F2 3. Determine the formulas: Mg2+ and N3- so magnesium nitride is Mg3N2 4. Write the formula equation: Mg + N2 Mg3N2 5. Balance the equation: 3Mg + N2 Mg3N2 Counting with Moles • Chemical reactions involve large numbers of very small particles – so chemist use a measuring unit called the mole to measure amounts. • One mole contains 6.02 x 1023 particles of a substance. (Avogadro’s number) Avogadro’s Number • http://www.youtube.com/watch?feature=pl ayer_embedded&v=TEl4jeETVmg Molar Mass • The mass of one mole of a substance is its molar mass. • The molar mass of an element = its atomic mass expressed in grams. • Examples: Ca = Fe = C= Zn = • The mass of one mole of a substance is its molar mass. • The molar mass of an element = its atomic mass expressed in grams. • Examples: Ca = 41.08 Fe = 55.847 C = 12.001 Zn = 65.38 Molar Mass • To find the molar mass of a compound or molecule: 1. Multiply the atomic mass of each atom x its subscript 2. Add all of these together for one compound or molecule • Example: H2O = H = 1.0x2 = 2.0 O = 15.9x1=15.9 The sum = 17.9g/mole Classifying Chemical Reactions • There are 5 general types of reactions: – Synthesis – Decomposition – Single Replacement – Double Replacement – Combustion Synthesis reaction • When two or more substances combine to form a single product For example: A + B AB 2H2 + O2 2H2O 2 or more simpler substances one complex substance Decomposition - opposite of synthesis • When one substance breaks down into 2 or more other substances. AB A + B 2H2O 2H2 + O2 One compound 2 or more simpler substances Single Replacement reaction • When one element replaces another element in a compound A + BC AC + B Element + compound new element + new compound Zn + CuSO4 ZnSO4 + Cu • Metals usually replace metals and non-metals usually replace non-metals. An element can replace any element that is below it in the list in a single replacement reaction. Double Replacement • When atoms or ions of two different compounds replace each other. AB + CD AD + CB 2 compounds 2 new compounds • Most double displacement reactions will not occur unless the reactants are dissolved in water so that they can become ions. • Not all combinations of reactants will undergo a reaction. Combustion Reaction • A substance reacts rapidly with oxygen CH4 + 2O2 CO2 + 2H2O Energy Changes in Reactions • The breaking of chemical bonds requires energy • The forming of chemical bonds releases energy • During a chemical reaction energy is either released or absorbed. Energy Changes in Reactions • During a chemical reaction energy is either released or absorbed. • Exothermic Reactions – release energy into the surroundings • Endothermic Reactions – absorb energy from the surroundings Energy Changes in Reactions • Exothermic Reactions – the energy released as bonds form in the products is greater than the energy required to break the bonds in the reactants. • Endothermic Reactions – more energy is required to break the bonds in the reactants than is released by bonds forming in the products. Reaction Rates • http://www.youtube.com/watch?feature=pl ayer_embedded&v=OttRV5ykP7A • Temperature • Surface Area • Stirring • Concentration • Catalysts