* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Non-Cardiac Surgery for Adults with CHD
Survey
Document related concepts
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Heart failure wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Cardiac surgery wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
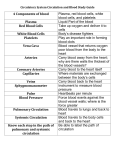
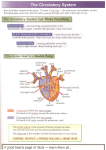
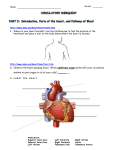
The Adult With Congentital Heart Disease James A. DiNardo, M.D., FAAP Senior Associate in Cardiac Anesthesia Children’s Hospital Boston Associate Professor of Anaesthesia Harvard Medical School A conservative estimate of the number of adult patients with congenital heart disease in the United States was 787,800 in the year 2000; approximately 117,000 of which have truly complex disease 1. Data from Quebec indicates that 49% of those alive in 2000 with severe congenital heart disease were adults 2. Management of these patients has been extensively addressed in a 5 part Task Force statement from the American College of Cardiology 1 3 4 5 6. Similar consensus documents have recently been generated by the Canadian Society of Cardiology 7 8 9 and the European Society of Cardiology 10. The issues which must be evaluated in all adult patients with congenital heart disease can be rightfully be characterized as residua (lesions for the most part intentionally left behind at the time of reparative surgery) or sequalae (necessary consequences of reparative operations or the natural history of the lesions) 11. These will be discussed in detail. Arrhythmias are common in these patients and have been recently reviewed extensively 12. Several lesions deserve particular attention as they are commonly encountered and are subject to a number of misconceptions: Tetralogy of Fallot (TOF) It is becoming increasing clear that the presence of pulmonary insufficiency resulting from trans-annular patch repair of TOF results in more long-term morbidity and mortality than previously appreciated 13 14 15. The pathophysiology is strikingly similar to that seen in chronic AI. Pulmonary valve replacement (surgical or percutanous) may be necessary in certain subsets of these patients. Arterial Switch Procedure (ASO) for D-TGA 1 This is a subset of patients with congenital heart disease is generally considered to be “cured”. Recent evidence demonstrates that these patients may have stress-induced perfusion defects and attenuated coronary blood flow reserve 16 perhaps related to sympathetic denervation 17. Fontan Physiology The essential function of the RV is not only to provide pulsatile flow through the pulmonary arterial system but to maintain a low pressure in the highly compliant systemic venous system, particularly the splanchnic bed 18. A single ventricle is capable of pumping through both the systemic and pulmonary circulations arranged in series. However, the lack of an RV reservoir requires that systemic venous pressure be elevated, as there is, in essence, a continuous column of blood from the aorta to the systemic capillaries, systemic veins, pulmonary capillaries, and finally the pulmonary veins. Normally 70% of the total blood volume is contained on the venous side of the circulation with the venous circulation having a capacitance 19 times that of the arterial circulation. Fontan patients adapt to reduce venous capacitance (reduce unstressed volume) so that elevated systemic venous pressure can be maintained with a normal systemic venous volume (increased stressed volume). This makes them particularly vulnerable to stimuli that reduce stressed volume such as increased venous capacitance (loss of muscle tone, venodilation from any source) and reductions in vascular volume (blood loss or dehydration). Figure 1. Pulmonary blood flow in the Fontan circulation is NOT “passive”. This common misconception inhibits the ability to fully understand Fontan physiology. Pulmonary blood flow in Fontan circulation is non-pulsatile, continuous flow; the systemic ventricle provides the driving energy for this flow. The Fontan circulation places the systemic and pulmonary vascular resistances in series with the systemic ventricle. Figures 2 and 3. Unfortunately, non-pulsatile pulmonary blood flow increases PVR by approximately 100% over than seen with pulsatile flow. Approximately 1/3 of the pulsatile energy generated by the RV is absorbed by the proximal pulmonary arterial system and redistributed in diastole to maintain recruitment of distal pulmonary vasculature. Loss of recruitment of distal pulmonary vasculature effectively reduces the area of the pulmonary vascular bed. This PVR increase is further exacerbated by the reduction in endothelial release of NO which accompanies long-term loss of pulsatile pulmonary blood flow 19. In addition, in the absence of pulsatility the total hydraulic power (mean + pulsatile flow) is converted into a pure pressure gradient, increasing the energy necessary for transmission of blood through the pulmonary circulation 20. The end result is an increase in the afterload on the single ventricle and an reduction in ventricular efficiency 21 22 23. This makes Fontan patients vulnerable to further increases in afterload (PVR or SVR) and to reductions in contractility. Positive pressure ventilation in Fontan patients is generally considered to be detrimental. The presumptive mechanism is the mechanical impediment of pulmonary blood flow with reduced delivery of blood to the systemic ventricle (reduced preload). Mechanical ventilation with reduced tidal volumes and low mean airway pressure may not be as detrimental to cardiac output in these patients as the factors generally associated with intubation and ventilation, specifically anesthesia/sedation (reduction of sympathetic output) and muscle relaxation (loss of muscular tone contribution to venous tone). Management of Fontan patients is further complicated by global impairment of cardiac 2 autonomic nervous activity with reduced heart rate variability and baroreceptor sensitivity 24. REFERENCES 1. Warnes CA, Liberthson R, Danielson GK, et al.: Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 2001; 37: 1170-5 2. Marelli AJ, Mackie AS, Ionescu-Ittu R, et al.: Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 2007; 115: 163-72 3. Foster E, Graham TP, Jr., Driscoll DJ, et al.: Task force 2: special health care needs of adults with congenital heart disease. J Am Coll Cardiol 2001; 37: 1176-83 4. Child JS, Collins-Nakai RL, Alpert JS, et al.: Task force 3: workforce description and educational requirements for the care of adults with congenital heart disease. J Am Coll Cardiol 2001; 37: 1183-7 5. Landzberg MJ, Murphy DJ, Jr., Davidson WR, Jr., et al.: Task force 4: organization of delivery systems for adults with congenital heart disease. J Am Coll Cardiol 2001; 37: 1187-93 6. Skorton DJ, Garson A, Jr., Allen HD, et al.: Task force 5: adults with congenital heart disease: access to care. J Am Coll Cardiol 2001; 37: 1193-8 7. Therrien J, Dore A, Gersony W, et al.: CCS Consensus Conference 2001 update: recommendations for the management of adults with congenital heart disease. Part I. Can J Cardiol 2001; 17: 940-59 8. Therrien J, Gatzoulis M, Graham T, et al.: Canadian Cardiovascular Society Consensus Conference 2001 update: Recommendations for the Management of Adults with Congenital Heart Disease--Part II. Can J Cardiol 2001; 17: 1029-50 9. Therrien J, Warnes C, Daliento L, et al.: Canadian Cardiovascular Society Consensus Conference 2001 update: recommendations for the management of adults with congenital heart disease part III. Can J Cardiol 2001; 17: 1135-58 10. Deanfield J, Thaulow E, Warnes C, et al.: Management of grown up congenital heart disease. Eur Heart J 2003; 24: 1035-84 11. Perloff JK, Warnes CA: Challenges posed by adults with repaired congenital heart disease. Circulation 2001; 103: 2637-43 12. Walsh EP, Cecchin F: Arrhythmias in adult patients with congenital heart disease. Circulation 2007; 115: 534-45 13. Geva T, Sandweiss BM, Gauvreau K, et al.: Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol 2004; 43: 1068-74 14. Knauth AL, Gauvreau K, Powell AJ, et al.: Ventricular Size and Function Assessed by Cardiac MRI Predict Major Adverse Clinical Outcomes Late After Tetralogy of Fallot Repair. Heart 2006 15. Geva T: Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2006: 11-22 3 16. Hauser M, Bengel FM, Hager A, et al.: Impaired myocardial blood flow and coronary flow reserve of the anatomical right systemic ventricle in patients with congenitally corrected transposition of the great arteries. Heart 2003; 89: 1231-5 17. Kondo C, Nakazawa M, Momma K, et al.: Sympathetic denervation and reinnervation after arterial switch operation for complete transposition. Circulation 1998; 97: 2414-9 18. Furey SA, 3rd, Zieske HA, Levy MN: The essential function of the right ventricle. Am Heart J 1984; 107: 404-10 19. Khambadkone S, Li J, de Leval MR, et al.: Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation 2003; 107: 3204-8 20. Mace L, Dervanian P, Bourriez A, et al.: Changes in venous return parameters associated with univentricular Fontan circulations. Am J Physiol Heart Circ Physiol 2000; 279: H2335-43 21. Senzaki H, Masutani S, Kobayashi J, et al.: Ventricular afterload and ventricular work in fontan circulation: comparison with normal two-ventricle circulation and single-ventricle circulation with blalock-taussig shunts. Circulation 2002; 105: 288592 22. Tanoue Y, Sese A, Imoto Y, et al.: Ventricular mechanics in the bidirectional glenn procedure and total cavopulmonary connection. Ann Thorac Surg 2003; 76: 562-6 23. Senzaki H, Masutani S, Ishido H, et al.: Cardiac rest and reserve function in patients with Fontan circulation. J Am Coll Cardiol 2006; 47: 2528-35 24. Davos CH, Francis DP, Leenarts MF, et al.: Global impairment of cardiac autonomic nervous activity late after the Fontan operation. Circulation 2003; 108 Suppl 1: II180-5 4 Figure 1. Elastic systemic illustrating the concepts of unstressed and stressed veins. Theunstressed volume is the volume necessary to fill the vessel to the point just below where intraluminal pressure is created. Unstressed volume does not generate a driving pressure in the circulation. Additional volume will create an intraluminal pressure; this is stressed volume. Unstressed volume can be reduced by reducing the size of the vessel (vasoconstriction) or increased by increasing the size of the vessel (vasodilatation). Stressed volume is the driving pressure in the circulation. Larger increases in pressure can be obtained for a given stressed volume by reducing vessel compliance. 5 Figure 2. Hydraulic circulation model. Normal series circulation is modeled with both pumps functioning and clamp B applied. Fontan physiology is modeled with the RV pump off and clamp B open. There is nothing “passive” about pulmonary blood flow. RS= systemic vascular resistance, RP= pulmonary vascular resistance, CAP= pulmonary arterial capacitance, CVP= pulmonary venous capacitance, CVS= systemic venous capacitance, CAS= systemic arterial capacitance 6 Figure 3. Acute transition from normal circulation to Fontan circulation using the model in Figure 2. At the arrow the RV pump is shunt down and the clamp B is removed (RV failure). Increased LV contractility and volume infusion are seen to correct the decreases in cardiac output and systemic blood pressure (PAS). PVS= systemic venous pressure, PVP= pulmonary venous pressure 7