* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Impact of Verapamil on Catecholamine Polymorphic Ventricular
Survey
Document related concepts
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Myocardial infarction wikipedia , lookup
Electrocardiography wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Transcript
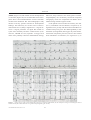
??? Case Report Acta Cardiol Sin 2011;27:115-9 The Impact of Verapamil on Catecholamine Polymorphic Ventricular Tachycardia Jen-Fu Liu Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a disease of mutation of family genes associated with exercise-induced syncope or sudden death. We report a case of a 27-year-old female with CPVT who had experienced several episodes of syncope and had been prescribed beta-blocker regularly. Sudden onset of ventricular arrhythmia occurred and the patient was resuscitated but remained in comatose state. During her hospitalization, the refractory intermittent ventricular arrhythmia was finally terminated adjuvantly by use of verapamil, while the current standard therapy for CPVT was definitely not useful. Therefore, we report this case accompanied by a review of the current literature. Key Words: Catecholamine polymorphic ventricular tachycardia · Sudden cardiac death · Verapamil INTRODUCTION death initially. This disorder is characterized by adrenergically induced ventricular arrhythmia, usually in the form of bidirectional or polymorphic VT in the absence of a structural heart disease or prolonged QT interval. Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a familial condition associated with exercise-induced syncope or sudden cardiac death in young adults. The disease is mostly caused by a mutation in the cardiac ryanodine channel gene that leads to abnormal increase in intracellular Ca2+ concentration, leading to arrhythmia due to a cascade of delayed after-depolarization and triggered activity. Current evidence suggests that the primary therapy for CPVT is beta blockade and placement of implantable cardioverter defibrillator (ICD). However, Mohamed et al. recently reported the case of a young girl with CPVT who died eventually despite beta-blockers and appropriate ICD therapies. CPVT is usually seen in children and young adults who present with exercise-induced syncope or sudden CLINICAL REPORT The 27-year-old female had initial episode of syncope at the age of 12 and three other episodes of syncope within the next 2 years. Each attack of syncope lasted from seconds to 1 or 2 minutes, followed by spontaneous recovery. In the past years, she had consulted several medical centers in Taiwan and America where indefinite arrhythmia was diagnosed and beta-blocker (kerlone) was prescribed for ordinary use. The patient was sent to a hospital in the neighborhood when she became unconscious and pulseless, having undetectable blood pressure and no spontaneous respirations. She was transferred to our hospital for personal reasons five days later. Upon arrival at the ER, her initial electrocardiographic survey (ECG) showed ventricular tachycardia/fibrillation (VT/VF). Sinus rhythm with complete right-bundle branch-like pattern Received: August 14, 2010 Accepted: December 30, 2010 Division of Cardiology, Tungs’ Taichung MetroHarbor Hospital, Jen-Teh Junior College of Medicine, Nursing and Management. Address correspondence and reprint requests to: Dr. Jen-Fu Liu, Division of Cardiology, Tungs’ Taichung MetroHarbor Hospital, Jen-Teh Junior College of Medicine, Nursing and Management, No. 699, Sec. 1, Chungchi Road, Wu-Chi township, Taichung, Taiwan. Tel: 886-4-2658-1919 ext. 4305; E-mail: [email protected] 115 Acta Cardiol Sin 2011;27:115 -9 Jen-Fu Liu Moreover, deep comatose state and hypoxic-ischemic encephalopathy was revealed by serial brain computed tomography, which was compatible with diffuse brain edema inciting ischemic-hypoxic insults. As the patient was moved into the coronary care unit (CCU), immediate oral beta-blocker, bisoprolol (1.25 mg/tablet) twice per day and intravenous amiodarone in continuous drip were given. We shifted gradually to oral mexiletine (150 mg/tablet) 600 mg per day with caution. Ventricular arrhythmia persisted, and we then shifted from amiodarone to lidocaine (2-3 mg/min) to try to (RBBB) (Figure 1A) and without obvious ST depression or elevation (Figure 1B) was recorded after three monophasic direct current defibrillations at 200 J and endotracheal tube intubation. Her echocardiographic study did not reveal any specific structural or hemodynamic findings. The laboratory test results were as follows: white blood cell: 15,060/ml, magnesium: 1.9 mg/dl, calcium: 7.3 mg/dl, potassium: 2.9 mg/dl. Her cardiac enzymes were markedly elevated: creatine kinase (CK): 5494 U/l, CK-MB: 32.8 U/l, troponin I: 32.0 mg/l, all of which were probably related to defibrillation therapy, A B Figure 1. (A) The onset of resting electrocardiogram at emergency room after defibrillation. (B) The subsequent resting electrocardiogram showed normal sinus rhythm without delta wave nor QT interval prolongation (QTc: 383 ms). Acta Cardiol Sin 2011;27:115 -9 116 Verapamil and Catecholamine Polymorphic Ventricular Tachycardia visiting time (Figures 2A, B, C). Verapamil, which is responsive to some ventricular arrhythmias, was used. 1 Fortunately, frequent VPEs disappeared regardless of further physical stimulation including pain and respiratory rehabilitation. No further episode of malignant cardiac dysarrhythmia occurred. In order to define the diagnosis and to optimize the dosage of beta-blocker, electrophysiologic study (EPS) with isoproterenol test was planned. Eventually, the EPS avoid long-QT phenomenon. A review of telemetry monitoring in the CCU revealed that sinus tachycardia was followed by bidirectional VT which was in turn converted into VF. CPVT was suspected due to typical bidirectional VT, which was probably triggered by sinus tachycardia. Although various anti-arrhythmic drugs were attempted, incremental bidirectional ventricular premature ectopics (VPEs) did show up and, in times, exacerbated to spontaneous VF on and off during family A B C D Figure 2. (A) The initial onset electrocardiogram showed multiple bidirectional ventricular premature ectopics (VPEs). (B) The subsequent electrocardiogram showed continuous bidirectional VPEs. (C) Spontaneous onset of ventricular fibrillation and self-limitation. (D) The sequencing figure showed a gene mutation(R2401 H) in the exon 47 of RyR2, heterozygous pattern at the seventh base from the left side. (arrow head) 117 Acta Cardiol Sin 2011;27:115 -9 Jen-Fu Liu therapy including aggressive suppression of polymorphic ventricular tachycardia (PVT) by anti-arrhythmia drugs such as calcium-channel blocker 5 and bilateral thoracic sympathectomy have been found to be effective.6 This patient’s ECG (Figure 1A) showed CRBBBlike pattern, and atypical Brugada syndrome was initially diagnosed. In patients who are suspected to have this disease, their arrhythmia may be detected by 24hour continuous monitoring ECG or induced by exercise treadmill testing. In this patient, transient increase in tachycardia may have been induced by the daily contact of family during visit in CCU, and, in turn, have transformed into bidirectional VPEs. Finally, CPVT might degenerate into PVT or VF. The activity of CPVT appears to be triggered by a burst in the sympathetic tone. Hence, the focus of treatment is to suppress adrenergic activity. Therefore, betablockers are effective for the acute phase and maintenance treatment.1 However, the symptoms recurred despite of the administration of sufficient dose of betablockers, lidocaine or amiodarone in this case. Frequent and easily-inducible VPEs suddenly disappeared after using verapamil. Normally, small calcium currents through L-type calcium channel in the cell membrane of cardiomyocytes trigger a large calcium flow from sarcroplasmic reticulum (SR). Verapamil can further reduce the amplitude of delayed depolarization (DAD) below the threshold required for triggering ventricular arrhythmia. The evidence of other calcium-channel blocker-related arrhythmia for CPVT seems insufficient. However, betablocker would prevent the adrenergic augmentation of the calcium flow of the SR through the genetically defective ryanodine channel.5,7 Beta-adrenergic receptor promote ryanodine channel phosphorylation and opening ryanodine channel boost the calcium flow from SR to depolarize the myocyte at the end of the action potential that creating DAD.8 Generally. CPVT was a heterogeneous genetic basis with gene mutation for ryanodine channel (CPVT1) and calcium-buffering protein calsequestrin (CPVT2). Screening for LQT genes, such as KCNJ2, may be considered in females with bidirectional/polymorphic VT when negative findings are obtained for the gene mutation of ryanodine channel and calcium-buffering protein calsequestrin.9 In summary, we have presented a novel ryanodine and ICD implant were not performed because of disagreement from the patient’s family. However, after a written informed consent was obtained for genetic analysis, genetic screening for the gene mutations of ryanodine channel and calcium buffering protein calsequestrin was performed using DNA extracted from the patients’ peripheral blood lymphocytes by the standard polymerase chain reaction (PCR) method. PCR analysis revealed an abnormal band in exon 47 of RyR2, which was not observed in unrelated healthy individuals. DNA sequencing confirmed a G to A transition leading to substitution of histidine to arginine 2401, R2401H (Fig 2D).2 No other mutation was observed in KCNQ1, KCNH2, SCN5A, KCNE1 and KCNE2 genes of this patient. Pedigree genetic screening for her mother, sister and brother showed no mutation either. Other family members were not available. DISCUSSION From 1975 to 1995, CPVT has been defined as the occurrence of malignant ventricular arrhythmia in response to exercise, isoproterenol infusion or any form of adrenergically-mediated stimulation.1,3 The characteristics of CPVT include normal QT-interval and bidirectional morphology of ventricular premature ectopic beats. There were some case reports among Chinese in Mainland China and Japanese, but none in Taiwanese. This report describes a young female who had previously received beta-blockers for undefined arrhythmia, ends up with the diagnosis of CPVT and treated completely with verapamil as adjuvant agent in spite of there being ICD. CPVT is a potentially lethal disease and difficult to diagnose, because of normal resting ECG and absence of obvious symptom in most afflicted individuals. A typical finding on ECG is VT with 180-degree alternation of the QRS axis. CPVT cannot be inducible by programmed electrical stimulation.1 Its mortality rates range from 20 to 31% in untreated patients and 10% in those who are treated with beta-blockers.4 Treatment options may consist of beta-blockade and ICD implantation but are not completely effective in some instances. Appropriate selection of candidates for ICD therapy is difficult as dependable risk stratification is lacking. Other adjuvant Acta Cardiol Sin 2011;27:115 -9 118 Verapamil and Catecholamine Polymorphic Ventricular Tachycardia Rhythm Society. Circulation 2006;114:E385-484. 2. Aizawa Y, Ueda K, Komura S, et al. A novel mutation in FKBP12.6 binding region of the human cardiac ryanodine receptor gene (R2401H) in a Japanese patient with catecholaminergic polymorphic ventricular tachycardia. Int J Cardiol 2005;99: 343-5. 3. Reid DS, Tynan M, Braidwood L, Fitzgerald GR. Bidirectional tachycardia in a child. A study using his bundle electrography. Br Heart J 1975;37:339-44. 4. Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002;106:69-74. 5. Rosso R, Kalman JM, Rogowski O, et al. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2007;4:1149-54. 6. Scott PA, Sandilands AJ, Morris GE, et al. Successful treatment of catecholaminergic polymorphic ventricular tachycardia with bilateral thoracoscopic sympathectomy. Heart Rhythm 2008;5: 1461-3. 7. Laitinen PJ, Brown KM, Piippo K, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 2001;103:485-90. 8. Chen YJ, Chen TC, Chen SA, et al. Cardiac cellular electrophysiology, voltage clamp, and patch clamp. Acta Cardiol Sin 2009;25:59-63. 9. Tester DJ, Arya P, Will M, et al. Genotypic heterogeneity and phenotypic mimicry among unrelated patients referred for catecholaminergic polymorphic ventricular tachycardia genetic testing. Heart Rhythm 2006;3:800-5. 10. Chan YH, Wu CT, Yeh YH, Kuo CT. Reappraisal of Luo-Rudy dynamic cell model. Acta Cardiol Sin 2010;26:69-80. 11. Chan YH, Wu LS, Yeh YH, et al. Possible targets of therapy for catecholaminergic polymorphic ventricular tachycardia: insight from a theoretical model. Circ J 2011 (in press). 12. Watanabe H, Chopra N, Laver D, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med 2009;15:380-3. channel mutation in a patient with CPVT. This R2401H mutation is located at the FKBP12.6 binding region of the ryanodine channel.2 The ventricular arrhythmia may be related to Ca2+ overload in the conducting tissue and precipitated by adrenergic stimuli or inhibited by betablocking drug. 10,11 Further studies focus on using flecainide to reduce release of Ca2+ from SR and to inhibit the Ca2+ signaling pathway mediated by ryanodine channel.12 But flecanide is not available at out hospital. As in our case, patients presenting with sudden cardiac arrest can be mistaken as idiopathic VF and suffer from the sequelae of resuscitation. Therefore, clinicians should carefully analyze the triggering factors of VF in healthy individuals. ACKNOWLEDGEMENT The PCR analysis for gene mutation was performed at Professor Lin-Ping Lai’s laboratory, National Taiwan University Hospital, Taipei, Taiwan. REFERENCES 1. Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart 119 Acta Cardiol Sin 2011;27:115 -9