* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download States of Matter
Chemical thermodynamics wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat equation wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Equipartition theorem wikipedia , lookup
Conservation of energy wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
R-value (insulation) wikipedia , lookup
Thermodynamic system wikipedia , lookup
Thermal radiation wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Heat capacity wikipedia , lookup
Adiabatic process wikipedia , lookup
Heat transfer wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermal conduction wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
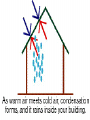
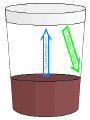
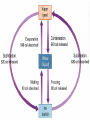
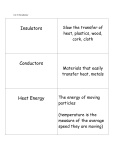
States of Matter Changes all around us What is Matter? • Matter is anything that has mass (atoms) and volume (takes up space). • EVERYTHING is made of matter. 3 States of Matter Water in all 3 states 4 States of Matter There are four states of matter: – – – – Solid Liquid Gas Plasma What Makes Something a Solid? Solids: – Retain their shape, – Molecules are close together, with strong force between molecules that holds the shape, – Molecules moving very slowly (vibrating) Solids What Makes Something a Liquid? Liquids: – Take the shape of its container, – Molecules close but not as tightly held as in solids, – Molecules move around each other. – Liquids can have different viscosities What is Viscosity? Viscosity = a liquids resistance to changing its shape. Thicker = higher viscosity Thinner = lower viscosity What is a Gas? • Gas: – takes the shape of its container, – molecules are very far apart and moving fast. Some Gas More Gas Lots and Lots of Gas Everything is in Motion All molecules are in motion Everything has Heat All molecules have heat. What is Heat? Heat is a form of energy WHAT IS HEAT? • Heat is: – a form of energy – the energy of vibrating molecules (Kinetic energy) – molecules are always moving (vibrating) therefore all matter has heat – the faster that molecules vibrate the more heat they have Heat is… • HEAT is the total kinetic energy of an object (Compared with Temperature which is the average Kinetic energy) ex. Block of ice vs. match Where Does Heat Come From? Sun, burning fuel, friction How Does Heat Move? Convection Convection is the molecule to molecule transfer of energy (requires matter) Conduction Conduction = substance in motion carries energy with it (requires matter) Radiation Radiation = photons travel through space from emitter to another point (does not require matter) How Do We Measure Heat? • Thermometer – measures the average Kinetic energy of the molecules in a substance. • Celsius = water freezes at 0oC and boils at 100oC • Farenheit = water freezes at 32oF and boils at 212oF. • Kelvin = begins at Absolute Zero = -275 celsius = limit of coldness = the lowest possible kinetic energy that molecules can have (Theoretic point) • What is Absolute Zero? • Absolute Zero = the temperature at which kinetic energy of molecules is zero, there is NO movement of the molecules. The Kinetic Molecular Theory • Kinetic Molecular Theory – explains thermal energy as the random movement of atoms or molecules; explains the properties of matter. • All matter is made of atoms (molecules) • The molecules are in constant motion (kinetic) • Motion and spacing of the molecules determines the state of matter • Temperature of the molecules determines the motion and thereby the state of matter. – More heat = more motion = more space Molecular Spacing and Heat Determine State of Matter or Phase • Solid = close molecules, slow, “cold” • Liquid = pretty close, faster, “warm” • Gas = far apart, fast, “hot” Phase Changes – Evaporation and Boiling • Liquid + heat Gas (Evaporation) – ex. Puddle disappearing on a hot day (Boiling) – Boiling = pressure of escaping water vapor = air pressure Evaporation is a Cooling Process Phase Changes - Melting • Solid + heat Liquid (Melting) – ex. Ice melting to water Phase Changes - Condensation • Gas – heat Liquid (Condensation) – ex. Liquid forming on the outside of a cold glass Phase Changes - Freezing • Liquid – heat Solid (Freezing) – ex. Water cooling and changing into ice Phase Changes - Sublimation • Solid + heat Gas (Sublimation) – ex. Dry ice, moth balls disappear over time Phase Changes - Deposition • Gas – heat Solid (Deposition) –ex. Snow, frost on windows, breath on cold day, frost in freezer Thermal Expansion • Thermal Expansion = the expansion that occurs as a substance is heated Review