* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download List 8 Vocabulary Cards - Endeavor Charter School
Survey
Document related concepts
Thermoregulation wikipedia , lookup
Insulated glazing wikipedia , lookup
Dynamic insulation wikipedia , lookup
Solar water heating wikipedia , lookup
Intercooler wikipedia , lookup
Solar air conditioning wikipedia , lookup
Heat exchanger wikipedia , lookup
Building insulation materials wikipedia , lookup
Heat equation wikipedia , lookup
Cogeneration wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
R-value (insulation) wikipedia , lookup
Transcript
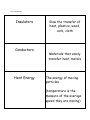
List 8 Vocabulary Insulators Conductors Heat Energy Slow the transfer of heat, plastics, wood, cork, cloth Materials that easily transfer heat, metals The energy of moving particles (temperature is the measure of the average speed they are moving) Conduction Convection Radiation Transfer of heat through touch/direct contact Transfer of heat through a liquid or a gas by rising and falling particles Transfer of heat through empty space, heat transfer from a distance Physical Change When a change in state happens, (melting, freezing, etc.) Mixing, but the item is still the same substance, reversible Examples include: Cutting string, crumpling paper, melting ice, evaporating water Chemical Change A change results in…. Heat, light, smoke, fizzing, color change, or smell Is not reversible Examples include: Rusting, burning something, cooking, spoiled food Mixture When two or more substances combine that do not form a new substance (this is a physical change, because the original substances keep their properties) 3 Main States of Matter Solid-tightly packed molecules with a defined volume and shape Liquid- Molecules that spread out a bit more and flow to take the shape of its container, but has a defined volume Gas- freely moving molecules with no defined volume or shape