* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thermochemistry
Solar air conditioning wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Process chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Marcus theory wikipedia , lookup
Click chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Heat transfer wikipedia , lookup
Thermometric titration wikipedia , lookup
Thermodynamics wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Stoichiometry wikipedia , lookup
George S. Hammond wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Transition state theory wikipedia , lookup
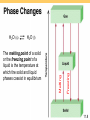
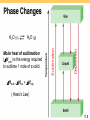
Thermochemistry Chapters 6 and 18 TWO Trends in Nature • Order Disorder • High energy Low energy Exothermic process is any process that gives off heat – transfers thermal energy from the system to the surroundings. 2H2 (g) + O2 (g) H2O (g) 2H2O (l) + energy H2O (l) + energy Endothermic process is any process in which heat has to be supplied to the system from the surroundings. energy + 2HgO (s) energy + H2O (s) 2Hg (l) + O2 (g) H2O (l) 6.2 Enthalpy (H) is used to quantify the heat flow into or out of a system in a process that occurs at constant pressure. DH = H (products) – H (reactants) DH = heat given off or absorbed during a reaction at constant pressure Hproducts < Hreactants DH < 0 Hproducts > Hreactants DH > 0 6.4 Thermochemical Equations Is DH negative or positive? System absorbs heat Endothermic DH > 0 6.01 kJ are absorbed for every 1 mole of ice that melts at 00C and 1 atm. H2O (s) H2O (l) DH = 6.01 kJ 6.4 Thermochemical Equations Is DH negative or positive? System gives off heat Exothermic DH < 0 890.4 kJ are released for every 1 mole of methane that is combusted at 250C and 1 atm. CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) DH = -890.4 kJ 6.4 Thermochemical Equations • The stoichiometric coefficients always refer to the number of moles of a substance H2O (s) • H2O (l) ΔH = 6.01 kJ If you reverse a reaction, the sign of DH changes H2O (l) • DH = 6.01 kJ/mol H2O (s) DH = -6.01 kJ If you multiply both sides of the equation by a factor n, then DH must change by the same factor n. 2H2O (s) 2H2O (l) DH = 2 mol x 6.01 kJ/mol = 12.0 kJ 6.4 Thermochemical Equations • The physical states of all reactants and products must be specified in thermochemical equations. H2O (s) H2O (l) DH = 6.01 kJ H2O (l) H2O (g) DH = 44.0 kJ How much heat is evolved when 266 g of white phosphorus (P4) burn in air? P4 (s) + 5O2 (g) 266 g P4 x P4O10 (s) 1 mol P4 123.9 g P4 x DHreaction = -3013 kJ 3013 kJ = 6470 kJ 1 mol P4 6.4 Standard enthalpy of formation (DHf0) is the heat change that results when one mole of a compound is formed from its elements at a pressure of 1 atm. The standard enthalpy of formation of any element in its most stable form is zero. DH0f (O2) = 0 DH0f (C, graphite) = 0 DH0f (O3) = 142 kJ/mol DH0f (C, diamond) = 1.90 kJ/mol 6.6 6.6 0 ) is the enthalpy of The standard enthalpy of reaction (DHrxn a reaction carried out at 1 atm. aA + bB cC + dD DH0rxn = [ cDH0f (C) + dDH0f (D) ] - [ aDH0f (A) + bDH0f (B) ] 0 (reactants) DH S DH0rxn = S DH0 (products) f f Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. (Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end.) 6.6 Benzene (C6H6) burns in air to produce carbon dioxide and liquid water. How much heat is released per mole of benzene combusted? The standard enthalpy of formation of benzene is 49.04 kJ/mol. 2C6H6 (l) + 15O2 (g) 12CO2 (g) + 6H2O (l) DH0rxn = S DH0 f(products) - S DH0 f(reactants) DH0rxn = [ 12DH0f (CO2) + 6DH0f (H2O)] - [ 2DH0f (C6H6)] DH0rxn = [ 12 × -393.5 + 6 × -285.8 ] – [ 2 × 49.04 ] = -6535 kJ -6535 kJ = - 3267 kJ/mol C6H6 2 mol 6.6 Calculate the standard enthalpy of formation of CS2 (l) given that: C(graphite) + O2 (g) CO2 (g) DH0rxn = -393.5 kJ S(rhombic) + O2 (g) CS2(l) + 3O2 (g) SO2 (g) DH0rxn = -296.1 kJ CO2 (g) + 2SO2 (g) 0 = -1072 kJ DHrxn 1. Write the enthalpy of formation reaction for CS2 C(graphite) + 2S(rhombic) CS2 (l) 2. Add the given rxns so that the result is the desired rxn. C(graphite) + O2 (g) 2S(rhombic) + 2O2 (g) + CO2(g) + 2SO2 (g) CO2 (g) DH0rxn = -393.5 kJ 2SO2 (g) DH0rxn = -296.1x2 kJ CS2 (l) + 3O2 (g) 0 = +1072 kJ DHrxn C(graphite) + 2S(rhombic) CS2 (l) 0 = -393.5 + (2x-296.1) + 1072 = 86.3 kJ DH rxn 6.6 Chemistry in Action: Fuel Values of Foods and Other Substances C6H12O6 (s) + 6O2 (g) 6CO2 (g) + 6H2O (l) DH = -2801 kJ/mol 1 cal = 4.184 J 1 Cal = 1000 cal = 4184 J The enthalpy of solution (DHsoln) is the heat generated or absorbed when a certain amount of solute dissolves in a certain amount of solvent. DHsoln = Hsoln - Hcomponents Which substance(s) could be used for melting ice? Which substance(s) could be used for a cold pack? 6.7 The Solution Process for NaCl DHsoln = Step 1 + Step 2 = 788 – 784 = 4 kJ/mol 6.7 Energy Diagrams Exothermic Endothermic (a) Activation energy (Ea) for the forward reaction 50 kJ/mol 300 kJ/mol (b) Activation energy (Ea) for the reverse reaction 150 kJ/mol 100 kJ/mol (c) Delta H -100 kJ/mol +200 kJ/mol Entropy (S) is a measure of the randomness or disorder of a system. order disorder S S If the change from initial to final results in an increase in randomness DS > 0 For any substance, the solid state is more ordered than the liquid state and the liquid state is more ordered than gas state Ssolid < Sliquid << Sgas H2O (s) H2O (l) DS > 0 18.3 First Law of Thermodynamics Energy can be converted from one form to another but energy cannot be created or destroyed. Second Law of Thermodynamics The entropy of the universe increases in a spontaneous process and remains unchanged in an equilibrium process. Spontaneous process: DSuniv = DSsys + DSsurr > 0 Equilibrium process: DSuniv = DSsys + DSsurr = 0 18.4 Entropy Changes in the System (DSsys) The standard entropy of reaction (DS0rxn ) is the entropy change for a reaction carried out at 1 atm and 250C. aA + bB DS0rxn = cC + dD [ cS0(C) + dS0(D) ] - [ aS0(A) + bS0(B) ] DS0rxn = S S0(products) - S S0(reactants) What is the standard entropy change for the following reaction at 250C? 2CO (g) + O2 (g) 2CO2 (g) S0(CO) = 197.9 J/K•mol S0(O2) = 205.0 J/K•mol S0(CO2) = 213.6 J/K•mol DS0rxn = 2 x S0(CO2) – [2 x S0(CO) + S0 (O2)] DS0rxn = 427.2 – [395.8 + 205.0] = -173.6 J/K•mol 18.4 Entropy Changes in the System (DSsys) When gases are produced (or consumed) • If a reaction produces more gas molecules than it consumes, DS0 > 0. • If the total number of gas molecules diminishes, DS0 < 0. • If there is no net change in the total number of gas molecules, then DS0 may be positive or negative BUT DS0 will be a small number. What is the sign of the entropy change for the following reaction? 2Zn (s) + O2 (g) 2ZnO (s) The total number of gas molecules goes down, DS is negative. 18.4 Spontaneous Physical and Chemical Processes • A waterfall runs downhill • A lump of sugar dissolves in a cup of coffee • At 1 atm, water freezes below 0 0C and ice melts above 0 0C • Heat flows from a hotter object to a colder object • A gas expands in an evacuated bulb • Iron exposed to oxygen and water forms rust spontaneous nonspontaneous 18.2 Gibbs Free Energy Spontaneous process: DSuniv = DSsys + DSsurr > 0 Equilibrium process: DSuniv = DSsys + DSsurr = 0 For a constant-temperature process: Gibbs free energy (G) DG = DHsys -TDSsys DG < 0 The reaction is spontaneous in the forward direction. DG > 0 The reaction is nonspontaneous as written. The reaction is spontaneous in the reverse direction. DG = 0 The reaction is at equilibrium. 18.5 DG = DH - TDS 18.5 The standard free-energy of reaction (DG0rxn) is the freeenergy change for a reaction when it occurs under standardstate conditions. aA + bB cC + dD 0 DGrxn = [cDG0f (C) + dDG0f (D) ] - [aDG0f (A) + bDG0f (B) ] 0 DGrxn = S DG0 f(products) - S DG0 f(reactants) Standard free energy of formation (DG0f ) is the free-energy change that occurs when 1 mole of the compound is formed from its elements in their standard states. DG0f of any element in its stable form is zero. 18.5 What is the standard free-energy change for the following reaction at 25 0C? 2C6H6 (l) + 15O2 (g) 12CO2 (g) + 6H2O (l) 0 DGrxn = S DG0 f(products) - S DG0 f (reactants) 0 DGrxn = [12DG0f (CO2) + 6DG0f (H2O)] - [ 2DG0f (C6H6)] 0 DGrxn = [ 12x–394.4 + 6x–237.2 ] – [ 2x124.5 ] = -6405 kJ Is the reaction spontaneous at 25 0C? DG0 = -6405 kJ < 0 spontaneous 18.5 Recap: Signs of Thermodynamic Values Negative Enthalpy (ΔH) Exothermic Positive Endothermic Entropy (ΔS) Less disorder More disorder Gibbs Free Energy (ΔG) Spontaneous Not spontaneous Gibbs Free Energy and Chemical Equilibrium DG = DG0 + RT lnQ R is the gas constant (8.314 J/K•mol) T is the absolute temperature (K) Q is the reaction quotient At Equilibrium DG = 0 Q=K 0 = DG0 + RT lnK DG0 = - RT lnK 18.6 DG0 = - RT lnK 18.6 The specific heat (s) [most books use lower case c] of a substance is the amount of heat (q) required to raise the temperature of one gram of the substance by one degree Celsius. The heat capacity (C) of a substance is the amount of heat (q) required to raise the temperature of a given quantity (m) of the substance by one degree Celsius. C = ms Heat (q) absorbed or released: q = msDt q = CDt Dt = tfinal - tinitial 6.5 How much heat is given off when an 869 g iron bar cools from 940C to 50C? s of Fe = 0.444 J/g • 0C Dt = tfinal – tinitial = 50C – 940C = -890C q = msDt = 869 g x 0.444 J/g • 0C x –890C = -34,000 J 6.5 Constant-Pressure Calorimetry qsys = qwater + qcal + qrxn qsys = 0 qrxn = - (qwater + qcal) qwater = msDt qcal = CcalDt Reaction at Constant P DH = qrxn No heat enters or leaves! 6.5 6.5 Phase Changes The boiling point is the temperature at which the (equilibrium) vapor pressure of a liquid is equal to the external pressure. The normal boiling point is the temperature at which a liquid boils when the external pressure is 1 atm. 11.8 The critical temperature (Tc) is the temperature above which the gas cannot be made to liquefy, no matter how great the applied pressure. The critical pressure (Pc) is the minimum pressure that must be applied to bring about liquefaction at the critical temperature. 11.8 Where’s Waldo? Can you find… The Triple Point? Critical pressure? Critical temperature? Where fusion occurs? Where vaporization occurs? Melting point (at 1 atm)? Carbon Dioxide Boiling point (at 6 atm)? The melting point of a solid or the freezing point of a liquid is the temperature at which the solid and liquid phases coexist in equilibrium Freezing H2O (l) Melting H2O (s) 11.8 Molar heat of sublimation (DHsub) is the energy required to sublime 1 mole of a solid. Deposition H2O (g) Sublimation H2O (s) DHsub = DHfus + DHvap ( Hess’s Law) 11.8 Molar heat of fusion (DHfus) is the energy required to melt 1 mole of a solid substance. 11.8 11.8 Sample Problem • How much heat is required to change 36 g of H2O from -8 deg C to 120 deg C? Step 1: Heat the ice Q=mcΔT Q = 36 g x 2.06 J/g deg C x 8 deg C = 593.28 J = 0.59 kJ Step 2: Convert the solid to liquid ΔH fusion Q = 2.0 mol x 6.01 kJ/mol = 12 kJ Step 3: Heat the liquid Q=mcΔT Q = 36g x 4.184 J/g deg C x 100 deg C = 15063 J = 15 kJ Sample Problem • How much heat is required to change 36 g of H2O from -8 deg C to 120 deg C? Step 4: Convert the liquid to gas Q = 2.0 mol x 44.01 kJ/mol = Step 5: Heat the gas ΔH vaporization 88 kJ Q=mcΔT Q = 36 g x 2.02 J/g deg C x 20 deg C = 1454.4 J = 1.5 kJ Now, add all the steps together 0.59 kJ + 12 kJ + 15 kJ + 88 kJ + 1.5 kJ = 118 kJ