* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Functional Characterization of the Arabidopsis Eukaryotic
Survey
Document related concepts
History of botany wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Plant nutrition wikipedia , lookup
Plant breeding wikipedia , lookup
Plant ecology wikipedia , lookup
Plant reproduction wikipedia , lookup
Plant physiology wikipedia , lookup
Plant morphology wikipedia , lookup
Perovskia atriplicifolia wikipedia , lookup
Transcript
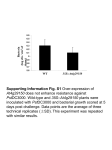
Functional Characterization of the Arabidopsis Eukaryotic Translation Initiation Factor 5A-2 That Plays a Crucial Role in Plant Growth and Development by Regulating Cell Division, Cell Growth, and Cell Death1[OA] Haizhong Feng, Qingguo Chen, Jian Feng, Jian Zhang, Xiaohui Yang, and Jianru Zuo* State Key Laboratory of Plant Genomics and National Center for Plant Gene Research, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100101, China The eukaryotic translation initiation factor 5A (eIF-5A) is a highly conserved protein found in all eukaryotic organisms. Although originally identified as a translation initiation factor, recent studies in mammalian and yeast (Saccharomyces cerevisiae) cells suggest that eIF-5A is mainly involved in RNA metabolism and trafficking, thereby regulating cell proliferation, cell growth, and programmed cell death. In higher plants, the physiological function of eIF-5A remains largely unknown. Here, we report the identification and characterization of an Arabidopsis (Arabidopsis thaliana) mutant fumonisin B1-resistant12 ( fbr12). The fbr12 mutant shows an antiapoptotic phenotype and has reduced dark-induced leaf senescence. Moreover, fbr12 displays severe defects in plant growth and development. The fbr12 mutant plant is extreme dwarf with substantially reduced size and number of all adult organs. During reproductive development, fbr12 causes abnormal development of floral organs and defective sporogenesis, leading to the abortion of both female and male germline cells. Microscopic studies revealed that these developmental defects are associated with abnormal cell division and cell growth. Genetic and molecular analyses indicated that FBR12 encodes a putative eIF-5A-2 protein. When expressed in a yeast mutant strain carrying a mutation in the eIF-5A gene, FBR12 cDNA is able to rescue the lethal phenotype of the yeast mutant, indicating that FBR12 is a functional eIF-5A. We propose that FBR12/eIF-5A-2 is fundamental for plant growth and development by regulating cell division, cell growth, and cell death. The eukaryotic translation initiation factor 5A (eIF5A) has been found in all eukaryotic organisms and is structurally and functionally conserved across different kingdoms. Biochemical and molecular studies revealed that eIF-5A is synthesized as an inactive precursor that is activated by a posttranslational modification mechanism. This modification is involved in a two-step reaction to convert an absolutely conserved Lys into a Hpu. The first reaction, catalyzed by deoxyhypusine synthase (DHS; EC 1.1.1.249), is to add a butylamine group derived from spermidine to the conserved Lys to form a deoxyhypusine. Subsequently, 1 This work was supported by the National Natural Science Foundation of China (NSFC; grant nos. 30330360 and 30221002), the Ministry of Science and Technology of China (grant no. 2006AA10A112), and the Chinese Academy of Sciences (grant no. KSCX2–YW–N–015). J.Z. is a recipient of the Outstanding Young Investigator Award of the NSFC (grant no. 30125025). * Corresponding author; e-mail [email protected]; fax 8610– 6487–3428. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jianru Zuo ([email protected]). [OA] Open Access articles can be viewed online without a subscription. www.plantphysiol.org/cgi/doi/10.1104/pp.107.098079 the deoxyhypusine residue is converted into a Hpu, catalyzed by deoxyhypusine hydroxylase (EC1.14.99.29; Park and Wolff, 1988; Wolff et al., 1990; Park et al., 1993, 1997). The hypusination of eIF-5A is essential for viability of yeast (Saccharomyces cerevisiae) cells (Kang et al., 1993) and highly specific, as demonstrated by the observation that a Lys-to-Arg mutation abolishes hypusination of eIF-5A (Jin et al., 2003). In addition, this unusual modification thus far has only been found in eIF-5A proteins (Park et al., 1997; Thompson et al., 2004). At the cellular level, Hpu-containing eIF-5A has been shown to be involved in the control of cell proliferation and apoptosis (Park et al., 1997; Tome and Gerner, 1997; Caraglia et al., 2001; Thompson et al., 2004). More recently, eIF-5A was found to function as a regulator of p53 and p53-dependent apoptosis (Li et al., 2004). The eIF-5A protein was initially identified as a translation initiation factor from rabbit reticulocytes in an in vitro assay (Kemper et al., 1976). The eIF-5A function in protein translation is partly supported by the observation that the putative translation initiation factor interacts with the ribosomal protein L5 (Schatz et al., 1998) and the translating 80S ribosomal complex (Jao and Chen, 2006). However, accumulating evidence suggests that eIF-5A is not required for protein synthesis in vivo because of the lack of correlation between eIF5A and the general protein synthesis (Park Plant Physiology, July 2007, Vol. 144, pp. 1531–1545, www.plantphysiol.org Ó 2007 American Society of Plant Biologists Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. 1531 Feng et al. et al., 1993, 1997; Kang and Hershey, 1994). Instead, Hpu-containing eIF-5A has been shown to stabilize mRNA and transport of specific subsets of mRNA from the nucleus to the cytoplasm (Bevec and Hauber, 1997; Zuk and Jacobson, 1998). In yeast, a temperaturesensitive mutant ts1159, which carries a mutation in the eIF-5A/TIF51A gene, causes the accumulation of uncapped mRNAs at the nonpermissive temperature, suggesting that eIF-5A is critical for mRNA turnover, possibly acting downstream of decapping (Zuk and Jacobson, 1998). The involvement of eIF-5A in the translocation of RNA has also been documented (Bevec and Hauber, 1997; Rosorius et al., 1999; Caraglia et al., 2001). In agreement with these observations, the solved crystal structure of eIF5A is characteristic of some RNA-binding proteins (Murzin, 1993), such as cold shock protein CspA (Schindelin et al., 1994) and the Escherichia coli translation initiation factor IF1 (Sette et al., 1997). Moreover, binding of eIF-5A to RNA requires Hpu on the protein and appears to be sequence specific to two core motifs of the targets (Xu and Chen, 2001). On the other hand, eIF5A was also found to interact with distinctive cellular proteins, including the exportin protein CHROMOSOME REGION MAINTE- NANCE1 (Rosorius et al., 1999), exportin 4 (Lipowsky et al., 2000), nucleoporins (Hofmann et al., 2001), tissue transglutaminase II (Singh et al., 1998), syntenin (Li et al., 2004), DHS, and Lia1 (for ligand of eIF5A; Thompson et al., 2003). On the basis of these observations, eIF5A was proposed to function as a bimodular protein capable of binding to both RNA and proteins (Liu et al., 1997; Jao and Chen, 2006), thus involved in multiple aspects of cellular signaling activities. In plants, genes encoding eIF-5A and DHS have been cloned from several species. Similar to its mammalian and yeast counterparts, a tomato (Lycopersicon esculentum) eIF-5A recombinant protein can be deoxyhypusine modified by a DHS recombinant protein (Wang et al., 2001). Whereas direct functional analysis of eIF-5A in planta has not been reported, suppression of DHS expression, which presumably causes partial inactivation of eIF-5A, has gained important information on the eIF-5A function. In Arabidopsis (Arabidopsis thaliana), constitutive overexpression of an antisense DHS cDNA causes delayed senescence and resistance to drought stress (Wang et al., 2003). Similarly, in tomato, overexpression of an antisense DHS construct results in delayed fruit softening and leaf senescence Figure 1. The fbr12 mutant shows an FB1-resistant phenotype and reduced PR gene expression. A, Two-week-old seedlings of wild type (Ws) and fbr12 germinated and grown in Murashige and Skoog medium in the absence or presence of 0.8 mM FB1. Bar 5 5 mm. B, Cell death induced by FB1 in wild-type and fbr12 plants. Three-week-old seedlings were treated with dimethyl sulfoxide (DMSO; 0.05%; control) or 2 mM FB1 for 24 h. Leaves were collected and stained with Trypan blue. Black staining represents dead or dying cells. C, Nuclear DNA fragmentation induced by FB1 in wild type and fbr12. Protoplasts prepared from wild-type and fbr12 leaves were treated with DMSO (0.002%; control) or 50 nM FB1 for 12 h and then analyzed by TUNEL staining as described in ‘‘Materials and Methods.’’ TUNEL-positive cells were counted under a fluorescent microscope (n . 1,000 in each experiment). Data presented were mean values of three independent experiments. Bars represent SEs. D, FB1-induced expression of PR1 and PR5 genes. Total RNA was prepared from 3-week-old seedlings treated with 3 mM FB1 for various times as indicated and used for northern-blot analysis. Each lane contains 15 mg of RNA. The blot was hybridized with a full-length PR1 or PR5 cDNA probe, respectively. 1532 Plant Physiol. Vol. 144, 2007 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Characterization of the Arabidopsis eIF-5A-2 Gene (Wang et al., 2005). In addition, because expression of both DHS and eIF-5A appears to be correlated with senescence or stress (Wang et al., 2001, 2003, 2005), it has been proposed that different isoforms of eIF-5A may facilitate the translation of mRNAs required for cell division and cell death, thereby regulating plant growth and development (Thompson et al., 2004). In this study, we report functional characterization of an Arabidopsis eIF-5A gene. A loss-of-function mutation in the eIF-5A-2 gene causes severe developmental defects throughout the life cycle, characteristics of abnormal cell division, cell growth, and cell death. These results demonstrate the biological importance of eIF-5A in plant growth and development. RESULTS Identification and Genetic Analysis of the fumonisin B1-resistant12 Mutant The fumonisin B1-resistant12 (fbr12) mutant was identified from a genetic screen of a T-DNA-mutagenized population of approximately 5,000 independent lines in the pga22 mutant background (Sun et al., 2003, Figure 2. Defective growth and development in fbr12. A, Four-day-old seedlings of wild type (Ws; left) and fbr12 (right) germinated and grown in Murashige and Skoog medium under continuous white light. No substantial difference between wild type and fbr12 is observed at this stage. Bar 5 1 mm. B, Ten-day-old seedlings of wild type (Ws; left) and fbr12 (right) germinated and grown in Murashige and Skoog medium under continuous white light. Bar 5 5 mm. C, Four-week-old plants of wild type (Ws; left) and fbr12 (right) germinated and grown in soil under continuous white light. Bar 5 5 mm. D, Rosette leaves collected from 4-week-old plants of wild type (Ws; top) and fbr12 (bottom) germinated and grown in soil under continuous white light. Bar 5 5 mm. E, Seven-week-old plants of wild type (Ws; left) and fbr12 (right) germinated and grown in soil under continuous white light. Bar 5 2 cm. F, Enlarged view of an fbr12 plant shown in E. The plant was germinated and grown in soil under continuous white light for approximately 7 weeks. Bar 5 2 cm. G, Comparison of wild-type (left) and fbr12 (right) floral inflorescences derived from 6-week-old plants germinated and grown in soil under continuous white light. Bar 5 2 mm. H, Analysis of growth rates of adult organs of wild-type and fbr12 plants germinated and grown in soil under continuous white light. Leaf initiation rate was calculated as the number of new leaves produced per day between the second and the seventh true leaves. Total number of leaves refers to rosette leaves 40 d after germination. Total number of flowers refers to flowers at stage 12 and above (flower development stages are defined according to Sanders et al. [1999]). At least 30 plants were analyzed in each experiment and average values were shown. Bars 5 SEs. Plant Physiol. Vol. 144, 2007 1533 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Feng et al. 2005). The pga22 mutant (Wassilewskija [Ws] accession) carries an estradiol-inducible T-DNA transcription activation tag (Zuo et al., 2000) upstream from the ISOPENTENYL TRANSFERASE8 (AtIPT8/PGA22) gene. Expression of the AtIPT8/PGA22 gene is highly inducible by the chemical inducer estradiol, which causes overproduction of cytokinin in planta. However, the pga22 mutant shows a phenotype indistinguishable from wild-type plants in the absence of estradiol (Sun et al., 2003). We have generated a T-DNA insertion population by transforming pga22 mutant plants with a second binary vector pTA231 (Sun et al., 2005). To screen fbr mutants, pooled T2 seeds (20 lines/ pool) were germinated on Murashige and Skoog medium (Murashige and Skoog, 1962) containing 0.8 mM fumonisin B1 (FB1). The fbr12 mutant was identified from this screen and showed strong resistance to FB1 (Fig. 1A). The original mutant (T3) was backcrossed with the wild type (Ws) twice and the resulting F2 or F3 progeny that did not contain the pga22 mutation were used for all experiments described below except for initial genetic analysis. Because fbr12 was infertile (see below), we crossed putative fbr12/1 plants (in the pga22 background) with the wild type (Ws). In F2 progeny derived from self-pollinated F1 plants, the FB1-resistant phenotype segregated in a 1:3 ratio (FB1 resistant:sensitive 5 72:225; x 2 5 0.055; P , 0.01). When grown under normal growth conditions in the absence of FB1, fbr12 displayed severe defects in plant growth and development (Fig. 1A; see below for details), and this pheno- type also segregated in a 1:3 ratio ( fbr12:wild type 5 75:288; x 2 5 3.58; P , 0.05). These results indicate that the fbr12 mutation is recessive in a single nuclear locus. Because of lethality of the mutation, fbr12 was maintained as heterozygous. The fbr12 Mutation Alters FB1-Mediated Programmed Cell Death To investigate the cellular and molecular alterations induced by FB1 in fbr12, we first compared toxininduced cell death by Trypan blue staining. In wildtype leaves, FB1 induced massive cell death. However, substantially reduced cell death was observed in fbr12 leaves (Fig. 1B). In some cases, no cell death was found in the mutant leaves treated by FB1 (data not shown). We next examined nuclear DNA fragmentation, a molecular hallmark of apoptotic cells, in protoplasts treated by FB1 using the TUNEL method. In untreated protoplasts derived from both the wild type and fbr12, TUNEL-positive signal was rarely detected. However, upon FB1 treatment, whereas more than 90% of wildtype protoplasts showed TUNEL-positive signals, ,25% of fbr12 protoplasts displayed distinctive positive signals under the identical assay conditions (Fig. 1C). At the molecular level, FB1 is known to induce the expression of PATHOGEN-RELATED (PR) genes, and the induction is compromised in fbr1 and fbr2 mutants (Stone et al., 2000). Similar to that of fbr1 and fbr2, FB1induced expression of PR1 and PR5 was reduced in fbr12 (Fig. 1D). Collectively, data presented in Figure 1 Figure 3. Developmental defects in the fbr12 floral organs. A, Comparison of wild-type and fbr12 flowers at different development stages. Bar 5 5 mm. B, Comparison of wild-type and fbr12 siliques. Bar 5 5 mm. C, Reduced numbers of floral organs in the fbr12 mutant. The x axis indicates distribution of floral organ numbers in the examined fbr12 flowers (n 5 105). Floral organ numbers of wild-type flowers are marked by asterisks above corresponding bars. 1534 Plant Physiol. Vol. 144, 2007 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Characterization of the Arabidopsis eIF-5A-2 Gene suggest that FB1-elicited PCD and/or defense response is altered by the fbr12 mutation. The fbr12 Mutation Causes Pleiotropic Phenotype during Plant Growth and Development The fbr12 mutant phenotype became apparent shortly after germination. Compared to the wild type, fbr12 was substantially smaller and more slender, with significantly shorter roots. However, all embryonic organs, including roots, hypocotyls, and cotyledons, appeared to be well defined (Fig. 2, A and B). The initiation of true leaves was substantially delayed in fbr12 (Fig. 2B). During later growth stages, fbr12 displayed a stunted phenotype (Fig. 2, C, E, and F), producing less rosette and cauline leaves that were smaller than those of the wild type (Fig. 2, D and E). In addition, fbr12 plants also had few flowers that were abnormally developed (Fig. 2, F and G). Overall, fbr12 affected the growth rate and organogenesis charac- teristics of dwarfism, a reduced leaf initiation rate and reduced numbers of adult organs (Fig. 2H). In fbr12, development of the floral organs was severely affected by the mutation. Compared to the wild type, fbr12 plants produced fewer flowers (Fig. 2, G and H) in which all floral organs displayed various developmental defects. In general, all fbr12 floral organs were smaller than those of the wild type (Fig. 3A). Compared to that of the wild type, sepals in fbr12 flowers were often misshapen and occasionally fused together. Petals in fbr12 flowers were smaller than that of the wild type (Fig. 3A). Stamens and stigmas appeared to be morphologically normal. However, two lateral stamens were usually absent in the mutant and the gynoecium stigmatic papillae was shorter than that of the wild type (see also below for more details on germline cell development). In wild-type Arabidopsis, a flower consists of four sepals, four petals, six stamens, and a carpel. Whereas a carpel was often found in fbr12 flowers, Figure 4. Delayed development of the SAM in fbr12. A to E, Light microscopy of longitudinal sections of wild-type and fbr12 seedlings. A, Fourday-old wild-type seedling. B, Six-day-old wildtype seedling. C, Four-day-old fbr12 seedling. The dome-structured SAM is smaller than that of wild type in the same stage shown in A, although apparent morphological alterations are not observed at this stage (see also Fig. 2A). D, Six-dayold fbr12 seedling. Compared to wild type at the same developmental stage shown in B, delayed initiation of true leaf projections is observed. E, Nine-day-old fbr12 seedling showing a development stage equivalent to that of 6-d-old wild-type seedlings shown in B. F to J, Scanning electron microscopy of the SAMs of wild type and fbr12. F, Four-day-old wild-type seedling. G, Six-day-old wild-type seedling. H, Four-day-old fbr12 seedling. I, Six-day-old fbr12 seedling showing a phenotype similar to that of 4-d-old wild type in F. J, Nine-day-old fbr12 seedling that appears to be in a developmental stage equivalent to that of 6-d-old wild-type seedlings shown in G. Bar 5 20 mm (A–E) and 50 mm (F–J). Plant Physiol. Vol. 144, 2007 1535 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Feng et al. other floral organ components had significantly reduced numbers in the mutant compared to the wild type (Fig. 3C). In particular, the number of petals and stamens was altered more dramatically by the fbr12 mutation, with ,30% and 10% of flowers producing correct numbers of these organs, respectively (Fig. 3C). Approximately 50% of flowers formed the correct number (four) of sepals. As a result of these defects, fbr12 siliques were markedly shorter and did not contain any seeds (Fig. 3B). The fbr12 Mutation Affects Cell Proliferation and Cell Growth To investigate the cellular basis of the fbr12 mutant phenotype, we analyzed the mutant by microscopy. Light microscopy revealed that the structure of the shoot apical meristem (SAM) was unaffected in fbr12, but SAM development was delayed compared to that of the wild type (Fig. 4, A–E). Notably, 9-d-old fbr12 SAM appeared to be equivalent to that of 6-d-old wild type. A similar observation was made by scanning electron microscopy (Fig. 4, F–J). These results suggest that the fbr12 mutation may cause a slow division or growth rate of the meristem cells. Transverse sections of stems revealed that fbr12, similar to that of the wild type, contained three distinctive layers of cell files that include, from outside to inside, the epidermis, cortex, and central cylinder. Development of epidermal cells appeared to be unaffected in the fbr12 mutant. This result suggests that radial patterning remains relatively normal in fbr12. In Figure 5. Cellular defects in the fbr12 mutant. A to D, Light microscopy of transverse sections of wild-type (A and C) and fbr12 (B and D) stems. C and D, Enlarged views of A and B, respectively. X, Xylem; P, phloem; C, cortex; E, epidermis. Bars 5 100 mm (A and B) and 30 mm (C and D). E, Analysis of cell numbers (y axis) in different tissues of wild-type and fbr12 stems. Data presented were average values obtained from 10 sections derived from different plants. Bars 5 SEs. 1536 Plant Physiol. Vol. 144, 2007 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Characterization of the Arabidopsis eIF-5A-2 Gene the cortex, however, cells were substantially enlarged in fbr12 compared to the wild type (Fig. 5, A–D). By contrast, the mutant had smaller cells in the central cylinder than those of the wild type (Fig. 5, A–D). The central cylinder contained the vascular bundles consisting of phloem and xylem. In xylem, fbr12 had an increased number of cells that were smaller, presumably representing a group of incompletely differentiated cells. Compared to that of the wild type, no distinctive cell layers were observed in the fbr12 phloem. Moreover, both the number and the size of the phloem cells were greatly reduced (Fig. 5, C and D). Quantitative analysis also indicated that fbr12 had increased cell numbers in xylem and parenchyma, but reduced cell numbers in phloem and cortex (Fig. 5E). Scanning electron microscopy indicated that, in fbr12 petals, whereas the cell number remains unaltered, the cell size is smaller than that of the wild type (Fig. 6, A and B). Quantitative analysis of cell numbers in petals revealed that the total cell numbers remained nearly unaltered, but the cell size was reduced in fbr12 (Fig. 6C), thus causing smaller petals. Collectively, these observations indicate that the fbr12 mutation affects cell proliferation, cell growth, and cell differentiation in both vegetative and reproductive organs/ tissues. FBR12 Is Essential for Male and Female Sporogenesis The fbr12 mutant could grow and develop into mature plants with significantly smaller size, which could not set any seeds. Reciprocal crosses between the wild type and fbr12 did not yield any F1 seeds, suggesting that the mutant was both male and female sterile. To reveal the cellular basis of the sterile phenotype, we followed the entire reproductive developmental stages by light microscopy and scanning electron microscopy. During male germline cell development, no apparent defects were found in fbr12 before stage 7 (anther development stages were defined according to Sanders et al. [1999]). In the wild type, at this stage, microspores are generated from pollen mother cells by meiosis and are confined as tetrads in a wall rich in callose, which, in turn, are contained in tapetum (Fig. 7A). In fbr12, tetrad cells appeared to be correctly generated (Fig. 7D). At stage 8, a wild-type pollen sac contains three layers of well-defined cell files, from outside to inside, epidermis, middle layer, and the tapetum. Inside the wild-type pollen sac, microspores are released from tetrads (Fig. 7B). At the same developmental stage, the middle layer and tapetum of the fbr12 pollen sac became disorganized with excessive numbers of cells that are irregularly shaped and stained darker compared to those in the wild type (Fig. 7, B and E). More severe defects were observed in later stages. In contrast to that of the wild type, no vacuole formation was found in fbr12 microspores; instead, microspores appeared to collapse and eventually formed dark-stained debris inside the disorganized tapetum (Fig. 7, C and F). Consequently, in mature anthers, no viable pollen grains were found in fbr12 by either toluidine blue O staining (Fig. 7, G and H) or scanning electron microscopy (Fig. 7, I–K). Figure 6. Reduced cell numbers in fbr12 petals. A and B, Scanning electron microscopy of wild-type (A) and fbr12 (B) petals. Compared to that of the wild type, the fbr12 petals have smaller cells. Bar 5 100 mm. C, Defective cell growth in fbr12 petals. In each image, the average values of the wild type (obtained by analyzing 20 petals derived from nine flowers) were set at 100% and the values of fbr12 relative to those of the wild type were given in the histogram. Bars 5 SEs. Plant Physiol. Vol. 144, 2007 1537 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Feng et al. Figure 7. Male-sterile phenotype of the fbr12 mutant. A to F, Light microscopy of cross-sections of pollen sacs derived from wildtype (A–C) and fbr12 (D–F) anthers. The images show anthers at different developmental stages (anther developmental stages are defined according to Sanders et al. [1999]): stage 7 (A and D), stage 8 (B and E), and stage 9 (C and F). At stage 7, no apparent developmental abnormality was observed in pollen sacs of fbr12 (D) compared to that of wild type (A). After entering stage 8, the tapetum of fbr12 had an excess of cell layers that encompassed degenerated pollen grains (E and F). E, Epidermis; En, endothecium; ML, middle layer; MSp, microspores; T, tapetum; Tds, tetrads. Bar 5 10 mm. G and H, Anthers of wild-type (G) and fbr12 (H) plants stained by toluidine blue O. Mature pollen grains are stained as dark blue. Bar 5 2 mm. I to K, Scanning electron microscopy of anthers of wild type (I) and fbr12 (J and K). Note the empty pollen sacs in fbr12 anthers. Bar 5 40 mm. 1538 Plant Physiol. Vol. 144, 2007 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Characterization of the Arabidopsis eIF-5A-2 Gene During female gametophyte development, the fbr12 mutation also appears to express after meiosis, approximately at stage 2-III (ovule development stages are defined according to Schneitz et al. [1995]). In the wild type at this stage, meiosis is completed to give rise to four megaspores of which only one survives and undergoes three rounds of nuclear divisions to eventually form a mature embryo sac. Meanwhile, the developing ovule grows toward the septum and the base of the carpel, whereas both the inner and outer integuments are initiated (Fig. 8, A and G). In fbr12, no apparent defects were observed at this stage and a megaspore appeared to be normally formed in the ovule (Fig. 8, D and H). In the wild type, whereas the inner integument continues to grow symmetrically, the outer integument starts asymmetric growth throughout development (Fig. 8B), eventually leading to the formation of a hook-shaped, mature embryo sac (Fig. 8, C and G). By contrast, both inner and outer integuments stopped further growth in fbr12 ovules (Fig. 8, E and H). Therefore, ovule development was completely arrested at this stage. We have also followed the entire reproductive development in fbr12/1 heterozygous plants. An fbr12/1 plant should give rise to haploid germline cells carrying a mutant or a wild-type allele, with approximately 50% of each genotype, respectively. On the other hand, the diploid sporophytic tissues (e.g. tapetum and ovules) should normally be developed because of the recessive nature of the mutation. No abnormally developed germline cells were observed in fbr12/1 plants, which showed no difference in fertility compared to wild type (data not shown). Moreover, in progeny derived from selfpollinated fbr12/1 plants, the mutation was segregated in a 1:3 ratio (mutant:wild type; see above) characteristic of sporophytic mutations (Yang et al., 1999; McCormick, 2004). These results suggest that the fbr12 mutation only affects sporogenesis, but not gametogenesis. Similarly, no abnormality was observed in developing embryos of fbr12/1 plants. Taken together, these results suggest that FBR12 is essential for sporophytic development, but dispensable for gametogenesis and embryogenesis, and that defective gametogenesis is likely caused by abnormal development of sporophytic tissues in the fbr12 mutant. Molecular Cloning of the FBR12 Gene The fbr12 mutant was identified from a T-DNAmutagenized population and the mutant genome appears to contain a single T-DNA insertion. We identified the genomic sequences flanking the left border by thermal asymmetric interlaced (TAIL)-PCR (Liu et al., 1995). DNA sequencing analysis indicated that the left border was inserted in the coding sequence region of At1g26630, 9 bp upstream from the stop codon, whereas the right border faced the 5#-end of the gene (Fig. 9A). Northern-blot analysis did not reveal any expression of At1g26630 in the mutant plants (Fig. 9B), suggesting that fbr12 is likely a null mutation. To verify the identity of FBR12, we carried out a molecular complementation experiment. A 2.5-kb wildtype genomic DNA fragment, which encompassed the promoter region, 5#-untranslated region (UTR), the coding sequence, and part of the 3#-UTR of At1g26630, was cloned into binary vector pER8 (Zuo et al., 2000). The resulting construct was then transformed into fbr12/1 heterozygous plants via Agrobacterium tumefaciensmediated transformation (Bechtold et al., 1993). Multiple independent transgenic lines were obtained by double selection with BASTA (carried by the fbr12 mutant) and hygromycin (carried by pER8). In the 20 tested T2 lines, all hygromycin-resistant plants displayed normal phenotype. In T3 lines derived from BASTA- and hygromycin-resistant T2 plants, we analyzed four families that did not show segregation of either the selective markers (BASTA and hygromycin) or the fbr12 phenotype. PCR analysis revealed that all tested plants (24) were homozygous for the T-DNA insertion at the At1g26630 locus. In these transgenic plants, the transgene fully rescued the developmental defects (Fig. 9, D and F) and restored the sensitivity of the mutant to FB1 (Fig. 9E). These data suggest that the At1g26630 transgene was able to fully complement the fbr12 mutant phenotype, thus representing FBR12. FBR12 Encodes a Functional eIF-5A-2 Database search identified a full-length FBR12 cDNA clone (accession no. NM_102425). Comparison of the cDNA and genomic sequences revealed an open reading frame (ORF) interrupted by four introns (Fig. 9A). The ORF encodes a polypeptide of 159 amino acid residues, with a predicted molecular mass of 17.1 kD and a pI of 5.8. Sequence comparison revealed that FBR12 encodes a putative eIF-5A-1 (accession no. NP_173985; also annotated by The Arabidopsis Information Resource [TAIR]) or eIF-5A-2 (accession nos. Q93VP3 and BE039424; Thompson et al., 2004). Hereafter, we refer to this protein as FBR12 or eIF-5A-2 according to Thompson et al. (2004). Consistent with the pleiotropic phenotype of fbr12, FBR12 appears to be ubiquitously expressed in all examined tissues and organs (Fig. 9C). Members of the eIF-5A family are highly conserved across different kingdoms of eukaryotic organism cells (Jenkins et al., 2001). The Arabidopsis genome contains two additional FBR12/eIF-5A-like genes, At1g69410 and At1g13950, which encode proteins sharing 86% and 82% identity with FBR12, respectively. In addition, FBR12 shares significant homology with representative eIF-5A proteins from other species, including rice (Oryza sativa; 79%; accession no. AAK1617679), mammals (human and mouse; 51%–54%), Drosophila (54%; accession no. AAG17032), Caenorhabditis elegans (55%; accession no. NP_499152), yeast (57%; accession no. NP_012581), and fission yeast (Schizosaccharomyces pombe; 52%; accession no. CAB16195). To functionally characterize FBR12, we carried out a genetic complementation experiment in a yeast mutant Plant Physiol. Vol. 144, 2007 1539 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Feng et al. Figure 8. Ovule development of fbr12 mutant plants. A to E, Scanning electron microscopy of wild-type and fbr12 ovules. A, Wild-type ovules at stage 2-III (ovule developmental stages are defined according to Schneitz et al. [1995]). The nucellus (nu), funiculus (fu), and inner (ii) and outer (oi) integument primordia are visible. B, Wild-type ovules at stage 3-I. The outer integument encompassed most of the inner integument and nucellus. C, Wild-type ovules at stage 4-I. The outer integument completely covers the inner integument and nucellus. D, fbr12 ovules at stage 2-III. Compared to the wild type, no apparent abnormality is observed. E, fbr12 ovules at stage 3-I. The outer integument and the inner integument stop development. F to H, Differential interference contrast observations of embryo sacs prepared from wild-type (F and G) and fbr12 (H) flower buds. Preparations were cleared by Herr’s solution (Herr, 1971). F, Wild-type ovule at stage 2-III. G, Wild-type ovule at stage 4-I. F, fbr12 ovule 2-III. fu, Funiculus; ii, inner integument; iip, inner integument primordium; nu, nucellus; oi, outer integument; oip, outer integument primordium; ov, ovule. Bar 5 100 mm (A–E) and 10 mm (F–H). strain PSY1249. This strain carries a temperaturesensitive mutation in an eIF-5A gene TIF51A that allows the mutant to grow only under the permissive temperature (25°C; Valentini et al., 2002). An FBR12 cDNA containing the entire ORF was cloned into a yeast expression vector under the control of the GAL1 promoter that can be induced by Gal, but repressed by Glc. The resulting construct pYES2-FBR12 was transformed into PSY1249. The transformed PSY1249 cells were able to grow at the nonpermissive temperature (36°C) in the presence of the Gal inducer, but not the Glc repressor (Fig. 10). Therefore, inducible expression of FBR12 cDNA is able to rescue the growth defects of PSY1249 caused by a mutation in the yeast eIF-5A gene. This result demonstrates that FBR12 is a functional eIF-5A. The fbr12 Mutation Delays Dark-Induced Leaf Senescence Previous studies showed that overexpression of antisense DHS in Arabidopsis and tomato resulted in delayed leaf senescence. Because DHS is essential for hypusination of eIF5A proteins, this transgenic phenotype was attributed to the inactivation of eIF5A proteins by knocking down DHS expression (Wang et al., 2003, 2005). To investigate the possible role of FBR12/eIF5A-2 in regulating senescence, we compared dark-induced leaf senescence in mutant and wild-type plants. Fully expanded leaves detached from fbr12 and wild-type plants were placed in the dark and their senescence rates were analyzed. Under assay conditions, leaves derived from wild-type plants showed an 1540 Plant Physiol. Vol. 144, 2007 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Characterization of the Arabidopsis eIF-5A-2 Gene growth conditions, the chlorophyll level was slightly lower in fbr12 leaves compared to that of the wild type. However, dark treatment caused a greater loss of chlorophyll in wild-type leaves (37% at day 4, 47% at day 7, and 80% at day 10) compared to that of fbr12 mutant leaves at the same stages (17% at day 4, 33% at day 7, and 48% at day 10; Fig. 11B). Taken together, these results suggest that FBR12/eIF5A-2 is involved in the regulation of senescence-type PCD in Arabidopsis. DISCUSSION Figure 9. Molecular characterization of the FBR12 gene. A, Schematic map showing the T-DNA insertion site in the fbr12 mutant genome. Black boxes denote exons and lines indicate untranslated sequences and introns. B, Northern-blot analysis of FBR12 expression in wild-type and fbr12 plants. Fifteen micrograms of total RNA prepared from 3-week-old plants were used for northern-blot analysis using an FBR12 cDNA probe. C, Quantitative analysis of FBR12 expression in different tissues/organs by real-time RT-PCR. Bars 5 SEs. D, Ten-day-old seedlings germinated and grown on Murashige and Skoog medium. From left to right, Wild type, fbr12, and fbr12 carrying an FBR12 transgene. Bar 5 5 mm. E, Fourteen-day-old seedlings germinated and grown on Murashige and Skoog medium containing 0.8 mM of FB1. From left to right, Wild type, fbr12, and fbr12 carrying an FBR12 transgene. Bar 5 5 mm. F, Seven-week-old plants germinated and grown in soil. From left to right, Wild type, fbr12, and fbr12 carrying an FBR12 transgene. Bar 5 2 cm. apparent dark-induced senescence syndrome at day 7 and became completely bleached at day 10. Under the identical assay conditions, leaves derived from fbr12 mutant plants displayed a slower senescence rate (Fig. 11A). To quantitatively analyze the senescence rate, we measured the chlorophyll levels in fully expanded leaves of wild type and fbr12. Under normal In this study, we present evidence showing that the Arabidopsis FBR12 gene encodes a functional eIF5A that is involved in the regulation of cell proliferation, cell growth, and cell death. FBR12 shares significant homology with eIF-5A proteins characterized across different kingdoms. Moreover, an FBR12 cDNA clone was able to complement the temperature-sensitive lethal phenotype of yeast PSY1249 cells carrying a mutation in the TIF51A/eIF-5A gene. These results demonstrate that FBR12 is a functional eIF-5A. The eIF-5A protein was originally characterized as a component of the translation initiation complex. However, recent studies suggest that this class of highly conserved proteins is involved in RNA metabolism and RNA trafficking (Bevec and Hauber, 1997; Zuk and Jacobson, 1998; Thompson et al., 2004), although very limited evidence is available for the proposed biochemical function of eIF-5A proteins. Nevertheless, because of its fundamental cellular function, eIF-5A has been shown to be required for growth and development in several organisms. In both yeast and mammalian cells, eIF-5A is essential for cell proliferation (Park et al., 1993, 1997; Kang and Hershey, 1994). In C. elegans, two copies of eIF-5A homologs, IFF-1 and IFF-2, were identified. Knockout of iff-2 results in slow Figure 10. FBR12 is a functional eIF-5A protein. An FBR12 cDNA fragment containing the entire ORF was placed under the control of the yeast GAL1 promoter in a pYES2 vector. The resulting construct pYESFBR12 was transformed into the yeast mutant strain PSY1249, which carries a temperature-sensitive mutation in the TIF51A/eIF-5A gene (Valentini et al., 2002). A wild-type yeast strain W303 (TIF51A) was used as a positive control and an empty pYES2 vector (vector) served as a negative control. The transformed cells were serially diluted and then inculcated on yeast peptone dextrose medium containing 2% (w/v) of Glc (Glu in figure) or 2% (w/v) of Gal. Plates were incubated at 24°C or 36°C for 2 (24°C) or 4 (36°C) d, respectively. Plant Physiol. Vol. 144, 2007 1541 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Feng et al. larval growth and disorganized somatic gonadal structures in hermaphrodites, whereas lack of IFF-1 activity causes sterility with underproliferated germline cells. Double mutants of iff-1/iff-2 displayed a slightly stronger phenotype than single mutants, but did not affect the viability of the animal (Hanazawa et al., 2004). In Arabidopsis, we found that a loss-of-function mutation in the FBR12/eIF-5A-2 gene causes more severe defects, including an underdeveloped SAM, reduced sizes and numbers of all adult organs, defective development of floral organs, and abnormal sporogenesis. Nevertheless, the loss of eIF-5A activity in both organisms shows some phenotypical similarities characteristic of slow growth and defects in reproductive development. On the other hand, we notice that gametogenesis and embryogenesis appear to be unaffected by the fbr12 mutation, suggestive of the presence of germline- and/or embryo-specific eIF-5A activity. A similar view is also shared by Thompson et al. (2004), who proposed that different isoforms of eIF-5A function distinctively in the regulation of cell divisions and cell death. This notion is reinforced by the observation that a null mutation in an Arabidopsis homologous gene eIF-5A-3 (SALK_022515) does not have substantial effects on plant growth and development (H. Feng, J. Feng, and J. Zuo, unpublished data). These results suggest that FBR12 represents major eIF-5A-2 activity during plant growth and development. The extreme dwarf phenotype of the fbr12 mutant may be caused by reduced cell division and/or cell growth. Microscopic studies suggest that both cell division and cell growth are affected by the fbr12 mutation. However, the regulatory roles of FBR12 in cellular activity appear to be cell type- or tissue-specific with distinctive mechanisms. In stem development, for example, no apparent abnormality was observed in the epidermal cell layer, but various defects were found in the cortex and the central cylinder. More strikingly, the FBR12 gene appears to function differently, characteristically by inhibiting cell growth in the cortex, but promoting cell growth in the central cylinder. In a similar mode, the fbr12 mutation causes increased cell numbers in xylem and parenchyma, but reduced cell numbers in phloem and cortex. These observations suggest that FBR12 regulates cell division and cell growth in a tissue- and development-specific manner. We notice that fbr12 shows some phenotypic similarity with fbr6, whose wild-type allele encodes a transcription activator AtSPL14 (Stone et al., 2005). In particular, both fbr6 and fbr12 affect vascular tissue development, suggesting that they may act in a linear pathway. It will be interesting to analyze the possible interaction of these two loci. In addition to its role in cell division and cell growth, FBR12 also appears to play a role in regulating cell death. Similar to other fbr mutants, fbr12 shows resistance to FB1 with substantially reduced cell death induced by the toxin. Compared to the wild type, fbr12 protoplasts showed substantially less DNA fragmentation induced by FB1, suggesting that FBR12/ Figure 11. Reduced dark-induced leaf senescence in fbr12. A, Fully expanded leaves detached from 4-week-old seedlings of wild type and fbr12 were kept at 22°C in the dark for different time (d) as indicated at the bottom of the image. Leaves were collected from 10 to 15 seedlings for each sample and the experiment was repeated three times. B, Measurement of the chlorophyll content in fully expanded leaves that were treated as described in A. Data presented were mean values from three independent experiments. Bars 5 SEs. eIF-5A-2, similar to its mammalian homologs, is likely involved in the regulation of apoptotic cell death. Because FB1 is known to inhibit ceramide synthase, thereby perturbing sphingolipid metabolism (Wang et al., 1991; Abbas et al., 1994; Gilchrist et al., 1994), FBR12 may be involved in the regulation of a subset of RNA responsible for sphingolipid metabolism or signaling. In addition to the antiapoptotic phenotype induced by FB1, fbr12 also shows delayed leaf senescence induced by dark, another form of PCD in plant cells. This phenotype is consistent with two earlier observations made in Arabidopsis (Wang et al., 2003) and tomato (Wang et al., 2005) in which antisense suppression of DHS, which presumably inactivates eIF-5A by blocking hypusination, leads to delayed senescence and tolerance to stresses. However, because fbr12 grows slower than wild-type plants, we could not exclude the possibility that delayed senescence in the mutant is partly attributed to slower initiation of the developmental program induced by dark. Nevertheless, in parallel to these findings in plants, the hypusination activity on eIF-5A was significantly reduced during senescence of IMR-90 human diploid fibroblasts (Chen and Chen, 1997). Taken together, these observations suggest that FBR12 and/or other eIF-5A genes may be directly or indirectly involved in the regulation of distinctive forms of cell death in plants. In conclusion, FBR12/eIF-5A-2 plays a critical role in plant growth and development by regulating cell division, cell growth, and cell death. Identification of the 1542 Plant Physiol. Vol. 144, 2007 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Characterization of the Arabidopsis eIF-5A-2 Gene fbr12 mutant provides unique materials to functionally characterize this class of highly conserved proteins in eukaryotic organisms. Clearly, identification and characterization of direct targets of FBR12/eIF-5A-2 will be critical to better understand its function. MATERIALS AND METHODS Plant Materials, Growth Conditions, and Genetic Screen for fbr Mutants The Ws ecotype of Arabidopsis (Arabidopsis thaliana) was used in this study unless otherwise indicated. Plants were grown under a 16-h-light/8-h-dark cycle (white light; 120 mmol m22 s21) at 22°C in soil or on Murashige and Skoog medium (13 Murashige and Skoog salts, 3% Suc, 0.8% agar) as described previously (Sun et al., 2003). A T-DNA-mutagenized population of approximately 5,000 independent lines was generated in the pga22 (Ws) background (Sun et al., 2003, 2005). These T-DNA insertion lines were screened for FB1-resistant fbr mutants as described (Stone et al., 2000). T2 seeds were germinated and grown on Murashige and Skoog medium containing 0.8 mM FB1 (purchased from Sigma) under continuous white light for 1 to 2 weeks. Putative fbr mutants were identified and then transferred onto fresh Murashige and Skoog medium without FB1. From this screen, two fbr mutants were identified. The fbr12 mutant (originally named p250) was outcrossed with wild-type plants (Ws) twice to segregate out the pga22 mutation, and F2 or F3 progeny in the Ws background lacking the pga22 mutation were used in all experiments. Protoplast Preparation, Detection of Nuclear DNA Fragmentation, and Detection of Cell Death Leaves were collected from 4- to 5-week-old seedlings and protoplasts were prepared according to Danon and Gallois (1998). In situ detection of nuclear DNA fragmentation was performed as described (Danon and Gallois, 1998) with minor modifications. The TUNEL reaction was carried out in an Eppendorf tube by using an in situ cell death detection kit (Roche Diagnostics) according to the manufacturer’s instructions. Nuclear DNA was stained with Hoechst 33342 (5 mg/mL; Sigma). After staining, the samples were mounted on a polylysine slide and visualized using a fluorescent microscope. Cell death was analyzed by Trypan blue staining as described (Mou et al., 2000). Light and Electron Microscopic Analyses Semithin sections were prepared and analyzed as described with minor modifications (Yang et al., 1999). Briefly, samples were fixed in 2.5% glutaraldehyde, 0.2 M sodium phosphate buffer, pH 7.2, at 4°C overnight and then postfixed in 1% osmium tetroxide for 2 h at 4°C. After dehydration in an acetone and graduated ethanol series, samples were embedded in Spurr’s resin (Sigma). Semithin sections (2 mm) were stained with 0.1% (w/v) toluidine blue O and observed under a light microscope. For differential interference contrast observations, young flower buds were cleared with Herr’s solution (Herr, 1971), and then analyzed under an inverted light microscope. Pollen grains were stained by 0.1% (w/v) toluidine blue O. For scanning electron microscopic analysis, samples were fixed, postfixed, and dehydrated as described above. After being critical point dried in liquid CO2 and mounted, samples were sputter-coated with gold in an E-100 ion sputter and then observed under a scanning electron microscope (model S-570; Hitachi). For comparison of cell size and numbers, the distal portion of the petal epidermis or stem sections was analyzed as described (Mizukami and Ma, 1992; Mizukami and Fischer, 2000). For statistical analysis, images were photographed with an Olympus digital camera and then analyzed using Image-Pro Plus 5.1. Statistical calculations were performed with Microsoft Excel. Analysis of Leaf Senescence and Measurement of Chlorophyll Fully expanded leaves collected from 4-week-old wild-type or fbr12 plants were used for the analysis of dark-induced leaf senescence essentially as described (Guo and Crawford, 2005). Total chlorophyll was extracted from dark-treated or not-treated leaves and analyzed as described (Lichtenthaler, 1987). Molecular Complementation of the fbr12 Mutant Phenotype The T-DNA-tagged genomic sequence in the fbr12 genome was identified by TAIL-PCR as previously described (Liu et al., 1995; Sun et al., 2003). For molecular complementation, a 2.5-kb FBR12 genomic clone was obtained by PCR using primer pairs (FBR12F1, 5#-CCTCGAGGTGGGGCCGCATGGAATGCA-3#; and FBR12B1, 5#-CACTAGTCACTCTTGAATCGTGCA-3#) and PWO DNA polymerase (Roche Diagnostics). The genomic clone included a 1.1-kb sequence upstream from the translation start codon and 0.3 kb of the 3#-UTR sequence. The PCR fragment, digested with XhoI and SpeI, was cloned into the same sites of pER8 (Zuo et al., 2000). The resulting construct was transformed into Agrobacterium tumefaciens strain GV3101, which was used for transformation of fbr12/1 heterozygous plants by vacuum infiltration (Bechtold et al., 1993). All other molecular manipulations were carried out according to standard methods (Sambrook and Russell, 2001). RNA northern-blot and real-time PCR analyses were carried out as described previously (Sun et al., 2003; Feng et al., 2006). Semiquantitative reverse transcription (RT)-PCR was performed as previously described (Sun et al., 2005). Actin8 (At1g49240) was used as an internal control in all assays. Primer pairs used in the RT-PCR analyses were (all sequences are from the 5#-end to the 3#-end): AT1G11980F, GAGTTCTTCTTCTTCCTCCA and AT1G11980R, GAAACCGGTCGAGCCAGTGA; AT3G59845F, GGTGAGGAATCTTTACTTGT and AT3G59845R, GTAGCTGAGAGGAACATTGA; AT5G03840F, CCATGAGCTCTTTCCTTCT and AT5G03840R, GTGTTGAAGTGATCTCTCGA; FBR12F, TCAAAAACCGTCCCTGCAAGGT and FBR12R, CATTTGGCGTGACCGTGCTT; and Actin8F, TTGCAGACCGTATGAGCAAAGAGA and Actin8R, TGGTGCCACGACCTTAATCTTCA. Genetic Complementation in Yeast Cells An FBR12 cDNA fragment was PCR amplified using primer pairs (AT1G26630F2, 5#-CCCCGGGATGTCTGACGACGAGCACCA-3#; and AT1G26630B2, 5#-CACTAGTTGACGTCCGTTGTCAAACTGGT-3#), and then cloned into a pGEM-T Easy vector (Promega). This cDNA fragment, containing the entire ORF of FBR12 and 32 bp of the 3#-UTR, was verified by DNA sequencing. The insert was released by SmaI and PstI digestion and cloned into the same sites of a pQE-82 L vector (Qiagen). The cDNA fragment released from pQE-FBR12 by SacI and SpeI was cloned into the SacI and XbaI of a pYES2 vector (Invitrogen) under the control of a GAL1 promoter. Therefore, FBR12 expression in yeast (Saccharomyces cerevisiae) cells is inducible by Gal and repressible by Glc (West et al., 1984; Giniger et al., 1985). Culture and transformation of yeast cells were carried out according to standard protocols (Ausubel et al., 1994). ACKNOWLEDGMENTS We would like to thank the Arabidopsis Biological Resource Center (ABRC) for providing seeds, and Dr. Allan Jacobson (University of Massachusetts Medical School) and Dr. Pamela A. Silver (Harvard Medical School) for providing yeast strains. We would also like to thank Dr. Yongbiao Xue, Dr. Shuhua Yang, and Dr. De Ye for critically reading the manuscript. We are grateful to Dr. Weicai Yang for valuable advice on microscopic studies. Received February 14, 2007; accepted May 16, 2007; published May 18, 2007. LITERATURE CITED Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L, Sessions AE, Wang E, Merrill AH Jr, Riley RT (1994) Fumonisin- and AAL-toxininduced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol 106: 1085–1093 Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1994) Current Protocols in Molecular Biology. Greene Publishing Associates and Wiley-Interscience, New York Plant Physiol. Vol. 144, 2007 1543 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Feng et al. Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci III 316: 1194–1199 Bevec D, Hauber J (1997) Eukaryotic initiation factor 5A activity and HIV-1 Rev function. Biol Signals 6: 124–133 Caraglia M, Marra M, Giuberti G, D’Alessandro AM, Budillon A, del Prete S, Lentini A, Beninati S, Abbruzzese A (2001) The role of eukaryotic initiation factor 5A in the control of cell proliferation and apoptosis. Amino Acids 20: 91–104 Chen ZP, Chen KY (1997) Dramatic attenuation of hypusine formation on eukaryotic initiation factor 5A during senescence of IMR-90 human diploid fibroblasts. J Cell Physiol 170: 248–254 Danon A, Gallois P (1998) UV-C radiation induces apoptotic-like changes in Arabidopsis thaliana. FEBS Lett 437: 131–136 Feng H, An F, Zhang S, Ji Z, Ling HQ, Zuo J (2006) Light-regulated, tissuespecific, and cell differentiation-specific expression of the Arabidopsis Fe(III)-chelate reductase gene AtFRO6. Plant Physiol 140: 1345–1354 Gilchrist DG, Wang H, Bostock RM (1994) Sphingosine-related mycotoxins in plant and animal diseases. Can J Bot 73: S459–S467 Giniger E, Barnum SM, Ptashne M (1985) Specific DNA binding of GAL4, a positive regulatory protein of yeast. Cell 40: 767–774 Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and darkinduced senescence. Plant Cell 17: 3436–3450 Hanazawa M, Kawasaki I, Kunitomo H, Gengyo-Ando K, Bennett KL, Mitani S, Iino Y (2004) The Caenorhabditis elegans eukaryotic initiation factor 5A homologue, IFF-1, is required for germ cell proliferation, gametogenesis and localization of the P-granule component PGL-1. Mech Dev 121: 213–224 Herr JM (1971) A new clearing-squash technique for the study of ovule development in angiosperms. Am J Bot 58: 785–790 Hofmann W, Reichart B, Ewald A, Muller E, Schmitt I, Stauber RH, Lottspeich F, Jockusch BM, Scheer U, Hauber J, et al (2001) Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin. J Cell Biol 152: 895–910 Jao DLE, Chen KY (2006) Tandem affinity purification revealed the hypusine-dependent binding of eukaryotic initiation factor 5A to the translating 80S ribosomal complex. J Cell Biochem 97: 583–598 Jenkins ZA, Haag PG, Johansson HE (2001) Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics 71: 101–109 Jin BF, He K, Wang HX, Wang J, Zhou T, Lan Y, Hu MR, Wei KH, Yang SC, Shen BF, et al (2003) Proteomic analysis of ubiquitin-proteasome effects: insight into the function of eukaryotic initiation factor 5A. Oncogene 22: 4819–4830 Kang HA, Hershey JW (1994) Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J Biol Chem 269: 3934–3940 Kang HA, Schwelberger HG, Hershey JW (1993) Translation initiation factor eIF-5A, the hypusine-containing protein, is phosphorylated on serine in Saccharomyces cerevisiae. J Biol Chem 268: 14750–14756 Kemper WM, Berry KW, Merrick WC (1976) Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Ba and M2Bb. J Biol Chem 251: 5551–5557 Li AL, Li HY, Jin BF, Ye QN, Zhou T, Yu XD, Pan X, Man JH, He K, Yu M, et al (2004) A novel eIF5A complex functions as a regulator of p53 and p53-dependent apoptosis. J Biol Chem 279: 49251–49258 Lichtenthaler HK (1987) Chlorophylls and carotenoids—pigments of photosynthetic biomembranes. Methods Enzymol 148: 350–382 Lipowsky G, Bischoff FR, Schwarzmaier P, Kraft R, Kostka S, Hartmann E, Kutay U, Gorlich D (2000) Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J 19: 4362–4371 Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8: 457–463 Liu YP, Nemeroff M, Yan YP, Chen KY (1997) Interaction of eukaryotic initiation factor 5A with the human immunodeficiency virus type 1 Rev response element RNA and U6 snRNA requires deoxyhypusine or hypusine modification. Biol Signals 6: 166–174 McCormick S (2004) Control of male gametophyte development. Plant Cell (Suppl) 16: S142–S153 Mizukami Y, Fischer RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci USA 97: 942–947 Mizukami Y, Ma H (1992) Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell 71: 119–131 Mou Z, He Y, Dai Y, Liu X, Li J (2000) Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12: 405–418 Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 Murzin AG (1993) OB (oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J 12: 861–867 Park MH, Lee YB, Joe YA (1997) Hypusine is essential for eukaryotic cell proliferation. Biol Signals 6: 115–123 Park MH, Wolff EC (1988) Cell-free synthesis of deoxyhypusine. Separation of protein substrate and enzyme and identification of 1,3diaminopropane as a product of spermidine cleavage. J Biol Chem 263: 15264–15269 Park MH, Wolff EC, Folk JE (1993) Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. Biofactors 4: 95–104 Rosorius O, Reichart B, Kratzer F, Heger P, Dabauvalle MC, Hauber J (1999) Nuclear pore localization and nucleocytoplasmic transport of eIF-5A: evidence for direct interaction with the export receptor CRM1. J Cell Sci 112: 2369–2380 Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11: 297–322 Schatz O, Oft M, Dascher C, Schebesta M, Rosorius O, Jaksche H, Dobrovnik M, Bevec D, Hauber J (1998) Interaction of the HIV-1 Rev cofactor eukaryotic initiation factor 5A with ribosomal protein L5. Proc Natl Acad Sci USA 95: 1607–1612 Schindelin H, Jiang W, Inouye M, Heinemann U (1994) Crystal structure of CspA, the major cold shock protein of Escherichia coli. Proc Natl Acad Sci USA 91: 5119–5123 Schneitz K, Hülskamp M, Pruitt RE (1995) Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. Plant J 7: 731–749 Sette M, van Tilborg P, Spurio R, Kaptein R, Paci M, Gualerzi CO, Boelens R (1997) The structure of the translational initiation factor IF1 from E. coli contains an oligomer-binding motif. EMBO J 16: 1436–1443 Singh US, Li Q, Cerione R (1998) Identification of the eukaryotic initiation factor 5A as a retinoic acid-stimulated cellular binding partner for tissue transglutaminase II. J Biol Chem 273: 1946–1950 Stone JM, Heard JE, Asai T, Ausubel FM (2000) Simulation of fungalmediated cell death by fumonisin B1 and selection of fumonisin B1-resistant ( fbr) Arabidopsis mutants. Plant Cell 12: 1811–1822 Stone JM, Liang X, Nekl ER, Stiers JJ (2005) Arabidopsis AtSPL14, a plantspecific SBP-domain transcription factor, participates in plant development and sensitivity to fumonisin B1. Plant J 41: 744–754 Sun J, Hirose N, Wang X, Wen P, Xue L, Sakakibara H, Zuo J (2005) Arabidopsis SOI33/AtENT8 gene encodes a putative equilibrative nucleoside transporter that is involved in cytokinin transport in planta. J Integr Plant Biol 47: 588–603 Sun J, Niu QW, Tarkowski P, Zheng B, Tarkowska D, Sandberg G, Chua NH, Zuo J (2003) The Arabidopsis AtIPT8/PGA22 gene encodes an isopentenyl transferase that is involved in de novo cytokinin biosynthesis. Plant Physiol 131: 167–176 Thompson GM, Cano VS, Valentini SR (2003) Mapping eIF5A binding sites for Dys1 and Lia1: in vivo evidence for regulation of eIF5A hypusination. FEBS Lett 555: 464–468 Thompson JE, Hopkins MT, Taylor C, Wang TW (2004) Regulation of senescence by eukaryotic translation initiation factor 5A: implications for plant growth and development. Trends Plant Sci 9: 174–179 Tome ME, Gerner EW (1997) Cellular eukaryotic initiation factor 5A content as a mediator of polyamine effects on growth and apoptosis. Biol Signals 6: 150–156 Valentini SR, Casolari JM, Oliveira CC, Silver PA, McBride AE (2002) Genetic interactions of yeast eukaryotic translation initiation factor 5A 1544 Plant Physiol. Vol. 144, 2007 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved. Characterization of the Arabidopsis eIF-5A-2 Gene (eIF5A) reveal connections to poly(A)-binding protein and protein kinase C signaling. Genetics 160: 393–405 Wang E, Norred WP, Bacon CW, Riley RT, Merrill AHJ (1991) Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem 266: 14486–14490 Wang TW, Lu L, Wang D, Thompson JE (2001) Isolation and characterization of senescence-induced cDNAs encoding deoxyhypusine synthase and eucaryotic translation initiation factor 5A from tomato. J Biol Chem 276: 17541–17549 Wang TW, Lu L, Zhang CG, Taylor C, Thompson JE (2003) Pleiotropic effects of suppressing deoxyhypusine synthase expression in Arabidopsis thaliana. Plant Mol Biol 52: 1223–1235 Wang TW, Zhang CG, Wu W, Nowack LM, Madey E, Thompson JE (2005) Antisense suppression of deoxyhypusine synthase in tomato delays fruit softening and alters growth and development. Plant Physiol 138: 1372–1382 West RWJ, Yocum RR, Ptashne M (1984) Saccharomyces cerevisiae GAL1- GAL10 divergent promoter region: location and function of the upstream activator sequence UASG. Mol Cell Biol 4: 2467–2478 Wolff EC, Park MH, Folk JE (1990) Cleavage of spermidine as the first step in deoxyhypusine synthesis. The role of NAD. J Biol Chem 265: 4793–4799 Xu A, Chen KY (2001) Hypusine is required for a sequence-specific interaction of eukaryotic initiation factor 5A with postsystematic evolution of ligands by exponential enrichment RNA. J Biol Chem 276: 2555–2561 Yang WC, Ye D, Xu J, Sundaresan V (1999) The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev 13: 2108–2117 Zuk D, Jacobson A (1998) A single amino acid substitution in yeast eIF-5A results in mRNA stabilization. EMBO J 17: 2914–2925 Zuo J, Niu QW, Chua NH (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273 Plant Physiol. Vol. 144, 2007 1545 Downloaded from on August 10, 2017 - Published by www.plantphysiol.org Copyright © 2007 American Society of Plant Biologists. All rights reserved.