* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Angiotensin II Increases Norepinephrine Release
Survey
Document related concepts
Nicotinic agonist wikipedia , lookup
Toxicodynamics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
NMDA receptor wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Norepinephrine wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Transcript
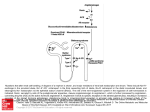
699 Angiotensin II Increases Norepinephrine Release From Atria by Acting on Angiotensin Subtype 1 Receptors Helmut Brasch, Ludwig Sieroslawski, Peter Dominiak Downloaded from http://hyper.ahajournals.org/ by guest on August 9, 2017 Norepinephrine stores in electrically driven guinea pig isolated atria were loaded with [3H] norepinephrine, and norepinephrine release was deduced from the radioactivity efflux. Electrical field stimulation of sympathetic nerve endings was applied during the refractory period of atrial contractions. The stimulation-induced release of norepinephrine was increased by angiotensin II (Ang II) (10"* to 10*"' mol/L) in a concentration-dependent manner. The maximum observed effect was a 55% augmentation. The effects of 10"7 and 10"6 mol/L Ang II were abolished by 10' 6 and 10"5 mol/L of the subtype 1 Ang II receptor antagonist losartan, respectively. Losartan by itself (10~6 mol/L) caused a 14% reduction of norepinephrine release. The subtype 2 Ang II receptor ligand PD 123319 (l-[[4-(dimethylamino)-3methylphenyl] methyl] -5-(diphenylacetyl)-4,5,6,7-tetrahydro-l//-imidazo [4,5 -c]pyridine-6-carboxylic acid ditrifluoroacetate) in a concentration of 10~4 mol/L had no detectable influence on transmitter release and did not antagonize the effect of Ang II. Angiotensin I (10~6 and 10"5 mol/L) increased norepinephrine release maximally by 23%. This effect was antagonized by 10"5 mol/L losartan and did not appear in the presence of 10~6 mol/L of the converting enzyme inhibitor ramiprilat. These results suggest that Ang II increases norepinephrine release by an activation of subtype 1 receptors, whereas angiotensin I is converted to Ang II to become effective. (Hypertension. 1993;22:699-704.) KEY WORDS • heart atrium • norepinephrine • angiotensin II • receptors, angiotensin • losartan I n plasma, angiotensin I (Ang I) is metabolized to the vasoconstricting octapeptide angiotensin II (Ang II) by a circulating converting enzyme. Additional amounts of Ang II are produced by local renin-angiotensin systems in a variety of tissues.'-3 In the heart, for instance, messenger RNA for the synthesis of renin has been detected, and a tissue-bound converting enzyme is also present, as well as Ang II, its precursor Ang I, and the metabolite angiotensin III. 3 5 Such a local renin-angiotensin system may be responsible for a variety of physiological and pathophysiological effects. For instance, Ang II increases protein synthesis in chick heart cells.6 Thus, the local production of the peptide may contribute to the development of cardiac hypertrophy, which can be prevented or reversed by converting enzyme inhibitors in experimental and human hypertrophy.7-8 Other effects of Ang II are a release of catecholamines from the adrenal medulla9'10 and from dopaminergic neurons in the central nervous system" and a facilitation of the release of norepinephrine from sympathetic nerve terminals in field-stimulated guinea pig, rat, and mouse atria. 1214 The positive inotropic effect of Ang II, which, according to Kuschinsky and Liillmann15 and Theyson and Received February 15, 1993; accepted in revised form June 16, 1993. From the Institute of Pharmacology, Medical University of Lubeck (FRG). Correspondence to Helmut Brasch, Institut fur Pharmakologie, Medizinische Universitat zu Lubeck, Ratzeburger Allee 160, D-23538 Lubeck, FRG. Klaus,16 does not appear in the presence of /3-adrenergic receptor antagonists, may therefore be caused by an increase of the local norepinephrine concentration. However, a direct influence of the peptide on calcium channels has also been suggested.1718 The first aim of this study was to determine whether the prejunctional effect of Ang II on norepinephrine release is mediated by Ang II subtype 1 (AT,) or subtype 2 (AT2) receptors in the isolated guinea pig atrium. To this end, the ATrselective receptor antagonist losartan19 and the AT2-selective receptor ligand211 PD 123319 (l-[[4-(dimethylamino)-3-methylphenyl]methyl]-5-(diphenylacetyl)4,5,6,7-tetrahydro-li/-imidazo[4,5-c]pyridine-6-carboxylic acid ditrifluoroacetate) were used. Further experiments with Ang I and the converting enzyme inhibitor ramiprilat suggest that a converting enzyme that continuously produces small amounts of endogenous angiotensin is active in the isolated atrium. Methods Experimental Procedure Two hundred male Pirbright white guinea pigs were used for the study. They were bought from the Lippische Versuchstierzuchtanstalt, Extertal, FRG, had free access to Altromin standard diet and tap water until used, and weighed 300 to 500 g on the day of experiments. The animals were killed by a blow on the head, and the hearts were rapidly removed. The left atrium was dissected out and mounted in a 10-mL organ bath that contained Krebs-Henseleit solution of the following com- 700 Hypertension Vol 22, No 5 November 1993 Downloaded from http://hyper.ahajournals.org/ by guest on August 9, 2017 position (mmol/L): NaCl, 117.6; KC1, 5.8; NaHCO3, 25; NaH2PO4,1.2; MgSO4,1.2; Cad 2 , 2.5; and glucose, 5.5 as well as 0.1 mmol/L Ca-EDTA and 0.06 mmol/L ascorbic acid as antioxidants. The solution was gassed with 95% O2 and 5% CO2. The atria were stimulated at a rate of 0.5 Hz through platinum electrodes in direct contact with the muscle with square pulses of 2-milliseconds duration and 1.5 times threshold voltage (Stimulator T, Hugo Sachs, Hugstetten, FRG). The resting tension was adjusted to 10 mN, and the force of contraction was registered isometrically with a K 30 force-displacement transducer (Hugo Sachs) on a Helcoscriptor HE 16 (Hellige, Freiburg, FRG). After an equilibration period of 15 minutes, 10 /xCi of 7-pHlnorepinephrine was added to establish a drug concentration between 5xlO~8 and 10"7 mol/L in the loading bath (calculated from the specific activity of 10 to 20 Ci/mmol, see below). After a loading period of 1 hour, the atria were carefully rinsed with Krebs-Henseleit solution and transferred to a 20-mL organ bath for the release experiments. During the release experiments, the atria were stimulated at a rate of 0.5 Hz as described above (continuous myocardial driving stimulation). The myocardial driving stimuli triggered a second Stimulator T that produced square pulses of 30-V strength and 0.2millisecond duration. To evoke norepinephrine release from sympathetic nerve endings, these supramaximal stimuli (field stimuli) were applied 10 milliseconds after the driving stimulus, ie, during the myocardial refractory period, through two parallel platinum electrodes (1.5 cm long, 0.5 mm in diameter, 1 cm apart, one on each side of the muscle). For 1 hour, only myocardial driving stimulation was used, and the bathing solution was changed every 10 minutes to wash away all [3H]norepinephrine that had not been taken up into the storage vesicles. Thereafter, a single field stimulus (2 milliseconds, 30 V) was applied during each refractory period for 10 minutes. Atria that did not give a significant positive inotropic response or that developed arrhythmia were discarded after this test stimulation period. For all other atria, the experiment proper began 15 minutes later. Cocaine (3xl0~ 5 mol/L) and atropine (10~7 mol/L) were added to the organ bath to prevent neuronal reuptake of norepinephrine and cholinergic effects of electrical field stimulation, respectively. Norepinephrine release from sympathetic nerve endings was stimulated three times in each atrium by applying one 0.2-millisecond field stimulus during each myocardial refractory period. The stimulation periods lasted 5 minutes each and were separated by 15-minute intervals. The first and second stimulation periods (Si and S2) served as individual controls, and the various test drugs were added 15 minutes before the third stimulation period (S3). Five minutes before the start and immediately after the end of S1; S2, and S3, the organ bath was filled with fresh Krebs-Henseleit solution. Samples (1 mL) of the bathing fluid were collected immediately before the beginning and at the end of each stimulation period and again 5 minutes later for the determination of radioactivity efflux. Calculations and Statistics The stimulation-induced radioactivity efflux from the atrium into the organ bath was taken as an indicator of the stimulation-induced norepinephrine release. For measurement of the radioactivity in the bathing fluid, the 1-mL samples were mixed with 9 mL scintillation fluid (Hydroluma, Baker, Deventer, the Netherlands) and counted for 10 minutes in a scintillation counter (BF 5003, Laboratorium Dr Bertold, Wildbad, FRG). Results are given as disintegrations per minute (dpm) per milliliter bathing fluid. The basal, unstimulated outflow of radioactivity was determined in the samples from the 5-minute collection periods immediately before and after each stimulation period. To obtain the stimulation-induced radioactivity efflux, we subtracted the mean basal outflow from the total radioactivity measured in the sample from the stimulation period. To compare the effects of the consecutive stimulation periods, we calculated the S2-S, and S3-S1 ratios for the stimulation-induced radioactivity release for each experiment. Their means and associated standard errors (n=7 to 11 muscles per group) are presented in the figures. Friedman's analysis of variance with ranks21 was used to compare the predrug (S2-S,) release ratios from the different groups of atria. To evaluate drug effects, we compared the S3-S| and S^-Si ratios from the same group of atria with the U test for paired data. A value of P^.05 for the two-tailed test was considered to indicate statistical significance throughout. Drugs The following drugs were used in the study: L-7[3H]norepinephrine hydrochloride (10 to 20 Ci/mmol, Du Pont de Nemours, Dreieich, FRG); Ca-EDTA, ascorbic acid, cocaine hydrochloride (all E Merck, Darmstadt, FRG); atropine sulfate (Pharma Hameln, Hameln, FRG); Ang I, Ang II (Sigma Chemie, Deisenhofen, FRG); losartan (kindly donated by Du Pont, Wilmington, Del); PD 123319 (provided by ParkeDavis, Mich); and ramiprilat (donation from Hoechst AG, Frankfurt, FRG). All drugs were dissolved in double-distilled water. Stock solutions of the peptides (1 mg/mL) were stored at -22°C until use. All other solutions were prepared daily, and microliter amounts were added to the organ bath. Results Predrug Control Values for Basal Outflow and Stimulated Release of Radioactivity Even when the sympathetic nerve endings were not depolarized by field stimulation, there was a continuous efflux of radioactivity from the atria. This basal outflow is thought to consist mainly of inactive norepinephrine metabolites.22-23 In 22 groups of atria, the mean unstimulated efflux measured for the 5-minute period immediately preceding S, ranged between 87±8 and 178±54 dpm/mL (lowest and highest value, respectively). It decreased by approximately 10% to 25% during the time course of the experiments and was not significantly influenced by any of the drugs under study. Field stimulation released additional amounts of radioactivity, and this stimulation-induced release can be regarded as a reliable indicator of an exocytotic release of norepinephrine from sympathetic nerve terminals. 2224 For the first stimulation period (S,), mean release rates between 116±16 and 263±69 dpm/mL were obtained (highest and lowest mean and SEM, Brasch et al Increase of Norepinephrine Release by Angiotensin Control P0 10"4 701 ANG I I 10"7 + P0 10"4 Downloaded from http://hyper.ahajournals.org/ by guest on August 9, 2017 FIG 1. Bar graph shows influence of angiotensin II (ANG II) on radioactivity release from left atria caused by three consecutive field-stimulation periods (S, to S3). Means of S2-S, release ratio (open columns) and ^-St release ratio (filled columns) are shown for each group of atria (n=8 to 9). Vertical bars indicate associated SEM. Angiotensin II was added 15 minutes before S,. *Significant difference (Ps.05, two-tailed) between S3-S, and SrS, ratio (U test for paired data). Drug concentrations are in moles per liter. FIG 3. Bar graph shows influence of angiotensin II (ANG II) in combination with PD 123319 (PD) on radioactivity release from left atria caused by three consecutive fieldstimulation periods (S, to S3). Means of S2-S! release ratio (open columns) and S3-S, release ratio (filled columns) are shown for each group of atria (n=8 to 9). Vertical bars indicate associated SEM. Drugs were added 15 minutes before S3. *Significant difference (Ps.05, two-tailed) between S3-S, and S2-S, ratio (U test for paired data). Drug concentrations are in moles per liter. respectively). Field stimulation also produced a significant positive inotropic effect, increasing the force of contraction by between 7.93±1.38 and 17.70±2.36 mN (highest and lowest mean value measured for Si). Although no drugs were added before S2, less radioactivity was released during the second stimulation period than during S, in most experiments, so that mean ^ - S , release ratios between 0.85±0.08 and 0.98±0.07 were calculated (Figs 1 through 5). ing from 0.81 ±0.09 to 0.89±0.07) were not significantly different from the corresponding S2-S, ratios (Figs 1 through 5). Control Experiments In control experiments, when no drug was added before S3, there was a further small decrease of the stimulation-induced release of radioactivity. But the resulting mean S3-S, ratios in five groups of atria (rang- Effects of Angiotensin II Ang II increased the stimulation-induced release of radioactivity in a concentration-dependent manner (Fig 1). The effect became apparent at a concentration of 3xlO" 8 mol/L and reached its maximum at a concentration of 10"6 mol/L. However, because of its large variability, the influence of 10"6 mol/L was not significantly different from the effect of 10"7 mol/L, and concentrations beyond 10~6 mol/L were not tested. The S3-S| release ratio of 1.28±0.19 obtained with 10~6 mol/L underestimates the maximum drug effect because FIG 2. Bar graph shows influence of angiotensin II (ANG II) in combination with losartan (LOS) on radioactivity release from left atria caused by three consecutive field-stimulation periods (S, to S3). Means of S2-S, release ratio (open columns) and S3-S, release ratio (filled columns) are shown for each group of atria (n=9). Vertical bars indicate associated SEM. Drugs were added 15 minutes before S 3 . *Signrficant difference (Ps.05, two-tailed) between S3-S, and S2-S, ratio (U test for paired data). Drug concentrations are in moles per liter. 3 Control LOS 10-« ANG I I IO- 7 ANG I I lO" 7 AW I I 10-* ANG I I 10"* + LOS I 0 " 7 + LOS 10-* + LOS 10" 6 + LOS I 0 " 5 702 Hypertension Vol 22, No 5 November 1993 FIG 4. Bar graph shows influence of angiotensin I (ANG I) and angiotensin I plus losartan (LOS) on radioactivity release from left atria caused by three consecutive field-stimulation periods (S, to S3). Means of S2-S, release ratio (open columns) and S3-S, release ratio (filled columns) are shown for each group of atria (n=7 to 11). Vertical bars indicate associated SEM. Drugs were added 15 minutes before S 3 . *Significant difference (Ps.05, two-tailed) between S3-S, and S2-S, ratio (U test for paired data). Drug concentrations are in moles per liter. Control ANG 1 io-» uc 1 10-5 Downloaded from http://hyper.ahajournals.org/ by guest on August 9, 2017 ANG 1 i<r* + LOS io-« the release during S2 was smaller than during S, (see above). Compared with S2, Ang II caused a 55% increase of the stimulation-induced release. The effect of Ang II was accompanied by a small (12%) augmentation of the positive inotropic effect of field stimulation, but the slight influences of the test drugs on atrial contractility are not further described or discussed. The nonpeptidic AT,-specific receptor antagonist losartan reduced the stimulatory influence of Ang II on transmitter release (Fig 2). A concentration of 1CT6 mol/L losartan was sufficient to suppress completely the effect of lfT7 mol/L Ang II, whereas 10"5 mol/L was needed to abolish the influence of 1CT6 mol/L of the peptide. By itself, 10"7 mol/L losartan had no effect on transmitter release (data not shown), but 10"6 mol/L Control RAM 10"* RAM 10"* + ANG I 10"* FIG 5. Bar graph shows influence of ramiprilat (RAM) and ramiprilat plus angiotensin I (ANG I) on radioactivity release from left atria caused by three consecutive fieldstimulation periods (S, to S3). Means of S2-S, release ratio (open columns) and S3-S, release ratio (filled columns) are shown for each group of atria (n=7 to 10). Vertical bars indicate associated SEM. Drugs were added 15 minutes before S3. *Significant difference (P^.05, two-tailed) between S3-S, and S2-S, ratio (U test for paired data). Drug concentrations are in moles per liter. ANG I i<r* + LOS 10-5 caused a 14% reduction of the stimulation-induced release of radioactivity (Fig 2). In contrast to losartan, the AT2-specific receptor ligand PD 123319 in concentrations up to 10"4 mol/L had no detectable influence on the effect of Ang II and was also without effect when applied alone (Fig 3). Effects of Angiotensin I The precursor of Ang II, Ang I, also increased the stimulation-induced release of radioactivity (Fig 4). However, the concentrations of the decapeptide needed to produce a significant change were approximately 10 times greater than those of the octapeptide. Although no clear-cut effect was seen in some preliminary experiments with 10~7 mol/L (not shown), 1CT6 and 10~5 mol/L produced a 23% and 18% increase, respectively. The effect of 1(T6 mol/L Ang I was abolished by 1CT5 mol/L losartan (Fig 4) and did not appear when 10~6 mol/L of the converting enzyme inhibitor ramiprilat was applied simultaneously (Fig 5). Ramiprilat by itself did not modify the stimulation-induced release of radioactivity. Discussion It has been concluded from in vivo experiments that Ang II increases the release of norepinephrine in the heart23; the effect has also been demonstrated directly in isolated heart tissues.1426 The present results are in line with previous studies that have demonstrated a 50% increase of transmitter release by 10"8 mol/L Ang II in guinea pig atria,12 a doubling by 3xl0~ 7 mol/L in rat atria,13 and a 50% increase by 10"8 to 10"7 mol/L in mouse atria.14 Obviously, the renin-angiotensin system modulates sympathetic tone not only by acting on the central nervous system11 or by increasing the release of catecholamines from the adrenal medulla9'10 but also by a local effect on sympathetic nerve endings in the tissues. As autoinhibition of norepinephrine release by stimulation of prejunctional a2-adrenergic receptors was not excluded, the true maximum of this local stimulatory effect may even have been underestimated in our experiments and in the earlier in vitro studies.1214 Clearly, the influence of Ang II on norepinephrine Brasch et al Downloaded from http://hyper.ahajournals.org/ by guest on August 9, 2017 release in the presence of a r adrenergic receptor antagonists deserves further investigation. In previous investigations,12-13 the effect of Ang II could be prevented by the nonselective angiotensin receptor antagonist saralasin. In our experiments, the effect of Ang II was abolished by losartan, a competitive Ang II antagonist with a high selectivity for AT, receptors.19-27 We did not construct complete concentrationresponse curves because the maximum increase of norepinephrine produced by the peptide was relatively small, but the results shown in Fig 2 are compatible with the assumption of a competitive antagonism between losartan and Ang II. This suggests that in guinea pig atria the influence of Ang II on the release of norepinephrine is mediated by prejunctional AT, receptors. Apparently, AT2 receptors are not involved, because the ligand PD 123319, which is selective for AT2 receptors,20 had no effect. AT2 receptors are present in the heart and account for up to 30% of the total angiotensin receptor population.19-28-29 Their physiological role is still uncertain, but AT2 receptors located on human cardiac fibroblasts have recently been shown to stimulate collagen synthesis.30 The precursor of Ang II, Ang I, was also able to increase the stimulation-induced release of norepinephrine. The antagonism by losartan shows that this effect also is caused by a stimulation of AT, receptors. Ang I has a low affinity to angiotensin receptors,31 but it is unlikely that it was the active compound in our experiments because its effect was not seen in the presence of the converting enzyme inhibitor ramiprilat. We conclude that Ang I must be converted to Ang II, which then acts as the receptor stimulant, as has already been suggested from experiments in guinea pig and rat atria12-14 and rat Langendorff-perfused hearts,32 in which the norepinephrine-releasing effect of Ang I could be prevented by saralasin, captopril, and ramiprilat. In these studies, Ang I was found to be 10 to 20 times less effective than Ang II, whereas both compounds were roughly equipotent in increasing the beating frequency of isolated guinea pig right atria.33 In our experiments we needed 10-fold greater concentrations of the decapeptide to produce roughly the same increase of transmitter release as with the octapeptide. Only approximately 7% of the administered dose of Ang I is metabolized to Ang II in perfusion experiments with isolated organs.3 This small conversion rate seems to be a reasonable explanation for the lower efficacy of Ang I but makes it difficult to understand why 10"5 mol/L losartan was needed to antagonize the effect of Ang I. As judged from the experiments with Ang II (Fig 2), 10~6 mol/L should have been sufficient. We cannot explain this discrepancy at the moment. The small reduction of norepinephrine release caused by losartan is an interesting finding. The drug is lipophilic (octanol/water partition coefficient of 7.77 at physiological pH; unpublished results) and, in principle, might act like a local anesthetic to inhibit membrane depolarization and subsequent exocytosis at the sympathetic nerve endings. However, the effect was fully overcome by increasing concentrations of Ang II (Fig 2), which makes such an unspecific influence unlikely. We suggest, therefore, that losartan antagonizes, at AT, receptors, the stimulating effect of small amounts of endogenous Ang II that are produced continuously by Increase of Norepinephrine Release by Angiotensin 703 the tissue converting enzyme. In contrast to our findings, no reduction of the transmitter release was observed in guinea pig atria in the presence of saralasin.12 But as saralasin is a partial agonist, the remaining intrinsic activity of this compound may have prevented the expected reduction. Perhaps more important, ramiprilat, in a concentration sufficient to inhibit the converting enzyme, did not reduce the transmitter release in our experiments, and several converting enzyme inhibitors, including captopril, were found to be ineffective by others.12-32 But this too does not necessarily contradict our hypothesis. An admittedly speculative explanation can be suggested for the lack of effect of the converting enzyme inhibitors. The converting enzyme is a rather unspecific dipeptidase that also inhibits the inactivation of bradykinin.34-35 Bradykinin is known to stimulate norepinephrine release from nerve terminals,36 and thus the stimulating influence of an increased bradykinin concentration and the inhibitory effect of a reduced Ang II concentration could cancel each other out. The fact that in pithed rats converting enzyme inhibitors do not change the stimulation-induced release of catecholamines37-38 and may even increase the norepinephrine spillover rate in conscious spontaneously hypertensive rats39 supports this idea. If Ang II produced in the tissue causes a significant modification of the norepinephrine release via prejunctional AT, receptors in the isolated atrium, a similar effect may occur also in vivo. Because of its implications for the local regulation of sympathetic tone, this idea clearly deserves further investigation. In conclusion, our results have shown that in fieldstimulated guinea pig atria, Ang II applied to the bathing solution increases the release of norepinephrine by an activation of prejunctional AT, receptors. Ang I is converted to Ang II to produce a similar effect. In addition, a continuous production of endogenous Ang II may be responsible for a certain degree of AT, receptor activation in the untreated atrium. Acknowledgment This work was supported by the Sandoz-Stiftung fur therapeutische Forschung. References 1. Gothert M, Kollecker P. Subendothelial ft-adrenoceptors in the rat vena cava: facilitation of noradrenaline release via local stimulation of angiotensin II synthesis. Naunyn Schmiedebergs Arch Pharmacol 1986^34:156-165. 2. Nakamaru M, Jackson EK, Inagami T. Beta-adrenoceptormediated release of angiotensin II from mesentenc arteries. Am J PhysioL 1986;250:H144-H148. 3. Lindpainter K, Wilhelm MJ, Jin M, Unger T, Lang RE, Scholkens BA, Ganten D. Tissue renin-angiotensin system: focus on the heart. J Hypertens. 1987;5(suppl 2):533-538. 4. Linz W, Schdlkens BA, Han YF. Beneficial effects of the converting enzyme inhibitor ramipril in ischemic rat hearts. J Cardiovasc Pharmacol. 1986;8(suppl 10):S91-S99. 5. Lindpaintner K, Ganten D. The cardiac renin angiotensin system: an appraisal of present experimental and clinical evidence. Circ Res. 1991;68:905-917. 6. Aceto JF, Baker KM. [Sar'Jangiotensin II receptor-mediated stimulation of protein synthesis in chick heart cells. Am J PhysioL 1990;258:H806-H813. 7. Linz W, Scholkens BA, Ganten D. Converting enzyme inhibition prevents the development and induces regression of cardiac hypertrophy in rats. Clin Exp Hypertens A. 1989;11:1325-1350. 8. Eichstaedt H, Danne O, Langer M, Cordes M, Schubert C, Felix R, Schmutzler H Regression of left ventricular hypertrophy under ramipril treatment investigated by nuclear magnetic resonance imaging. J Cardiovasc Pharmacol 1989;13(suppl 3):S75-S8O. 704 Hypertension Vol 22, No 5 November 1993 Downloaded from http://hyper.ahajournals.org/ by guest on August 9, 2017 9. Zimmermann BG, Gomer SK, Liao JC. Action of angiotensin on vascular adrenergic nerve endings: facilitation of norepinephrine release. Fed Proc. 1972;31:1344-1350. 10. Peach MJ. Renin-angiotensin system: biochemistry and mechanism of action. Physiol Rev. 1977;57:313-370. 11. Badoer E, Wiirth H, Tiirck D, Quadri F, Itoi K, Dominiak P, Unger T. Selective local action of angiotensin II on dopaminergic neurons in the rat hypothalamus in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:31-35. 12. Ziogas J, Story DF, Rand MJ. Effects of locally generated angiotensin II on noradrenergic transmission in guinea-pig isolated atria. Eur J Pharmacol. 1984; 106:11-18. 13. Mian MA, Majewski H, Rand MJ. Facilitation of noradrenaline release by isoprenaline in rat isolated atria does not involve angiotensin II formation. Clin Exp Pharmacol Physiol. 1989;16:905-911. 14. Musgrave IF, Majewski H. Effect of phorbol ester and pertussis toxin on the enhancement of noradrenaline release by angiotensin II in mouse atria. Br J Pharmacol. 1989;96:609-616. 15. Kuschinsky G, Liillmann H. Uber die Wirkungvon synthetischem Hypertensin auf Kammer- und Vorhofmuskulatur der Katze. Klin Wochenschr. 1959;37:928-931. 16. Theyson M, Klaus W. Uber den Mechanismus der positiv inotropen Angiotensinwirkung am isolierten Herzvorhofpraparat. Naunyn Schmiedebergs Arch Pharmacol. 1966;225:85-86. 17. Kass RS, Blair ML. Effects of angiotensin II on membrane current in cardiac Purkinje fibers. J Mol Cell Cardiol. 1981;13:797-809. 18. Baker KM, Singer HA, Aceto JF. Angiotensin II receptormediated stimulation of cytosolic-free calcium and inositol phosphates in chick myocytes. J Pharmacol Exp Ther. 1989;251:578-585. 19. Chiu AT, Herblin WF, McCall DE, Ardecky RJ, Carini DJ, Duncia JV, Pease LJ, Wong PC, Wexler RR, Johnson AL, Timmermanns PBMWM. Identification of angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989;165:196-203. 20. Dudley DT, Panek RL, Major TC, Lu GH, Bruns RF, Klinkefus BA, Hodges JC, Weishaar RE. Subclasses of angiotensin II binding sites and their functional significance. Mol Pharmacol. I99O;38: 370-377. 21. Bortz J, Lienert GA, Boehnke K. Verteilungsfreie Methoden in der Biostatistik. New York, NY: Springer-Verlag; 1990. 22. Marshall I. Stimulation-evoked release of 3H-noradrenaline by 1, 10 or 100 pulses and its modification through presynaptic «2-adrenoceptors. Br J Pharmacol. 1983;78:221-231. 23. Angus JA, Bobik A, Jackman GP, Kopin IJ, Korner PI. Role of auto-inhibitory feedback in cardiac sympathetic transmission assessed by simultaneous measurements of changes in 'H-efflux and atrial rate in guinea-pig atrium. Br J Pharmacol. 1984;81: 201-214. 24. Stjarne L, Astrand P. Relative pre- and postjunctional roles of noradrenaline and adenosine 5'-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1985; 14:929-946. 25. Krasney JA, Hogan PM, Lowe RF, Youmans WB. Peripheral adrenergic basis for cardioaccelerator action of angiotensin. Am J Physiol. 1966;211:1447-1450. 26. Starke K, Werner U, Schumann HJ. Wirkung von Angiotensin auf Funktion und Noradrenalinabgabe isolierter Kaninchenherzen in Ruhe und bei Sympathikusreizung. Naunyn Schmiedebergs Arch Pharmacol. 1969;265:170-186. 27. Wong PC, Price WA, Chiu AT, Carini DJ, Duncia JV, Johnson AL, Wexler RR, Timmermanns PBMWM. Nonpeptide angiotensin II receptor antagonists: studies with EXP 9270 and DuP 753. Hypertension. 1990; 15:823-834. 28. Whitebread S, Mele M, Kamber B, De Gasparo M. Preliminary biochemical characterisation of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989; 163:284-291. 29. Scott AL, Chang RSL, Lotti VJ, Siegl PKS. Cardiac angiotensin receptors: effects of selective angiotensin II receptor antagonists, DUP 753 and PD 121981, in rabbit heart. J Pharmacol Exp Ther. 1992;261:931-935. 30. Brilla CG. Angiotensin II type 2 receptor-mediated stimulation of collagen synthesis in human cardiac fibroblasts. Circulation. 1992; 86(suppl I):I-89. Abstract. 31. Unger T, Gohlke P, GruberMG. Converting enzyme inhibitors. In: Ganten D, Mulrow PJ, eds. Pharmacology ofAntihypertensive Therapeutics. Berlin/Heidelberg, FRG: Springer-Verlag; 1990:377-481. 32. Richardt G, Mayer E, Schomig, A: Role of angiotensin and sodium intake in cardiac noradrenaline release. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:297-301. 33. Nakashima A, Angus JA, Johnston CI. Chronotropic effects of angiotensin I, angiotensin II, bradykinin and vasopressin in guinea-pig atria. Eur J Pharmacol. 1982;81:479-485. 34. Graf K, Bossaller C, Auch-Schwelk W, Grafe M, Baumgarten C, Fleck E. ACE-inhibitors diminish the degradation of bradykinin and enhance endothelium-dependent relaxations in isolated coronary arteries. Pharm Pharmacol Lett. 1991; 1:71-73. 35. Wiemer G, Scholkens BA, Becker RHA, Busse R. Ramiprilat enhances endothelial autacoid formation by inhibiting breakdown of endothelium-derived bradykinin. Hypertension. 1991; 18: 558-563. 36. Dominiak P, Simon M, Blochl A, Brenner P. Changes in peripheral sympathetic outflow of pithed rats after bradykinin and des-argbradykinin infusions: influence of converting-enzyme inhibition. J Cardiovasc Pharmacol. 1992;20(suppl 9):S35-S38. 37. Dominiak P, Elfrath A, Tiirck D. Effects of chronic treatment with ramipril, a new ACE blocking agent, on presynaptic sympathetic nervous system of SHR. Clin Exp Hypertens A. 1987;9:369-373. 38. Dominiak P, Elfrath A, Tiirck D. Biosynthesis of catecholamines and sympathetic outflow in spontaneously hypertensive rats (SHR) after chronic treatment with CE-blocking agents. J Cardiovasc Pharmacol. 1987;10(suppl 7):S122-S124. 39. Kuo YJJ, Keeton TK. Captopril increases norepinephrine spillover rate in conscious spontaneously hypertensive rats. J Pharmacol Exp Ther. 1991;258:223-231. Angiotensin II increases norepinephrine release from atria by acting on angiotensin subtype 1 receptors. H Brasch, L Sieroslawski and P Dominiak Downloaded from http://hyper.ahajournals.org/ by guest on August 9, 2017 Hypertension. 1993;22:699-704 doi: 10.1161/01.HYP.22.5.699 Hypertension is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 1993 American Heart Association, Inc. All rights reserved. Print ISSN: 0194-911X. Online ISSN: 1524-4563 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://hyper.ahajournals.org/content/22/5/699 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Hypertension can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Hypertension is online at: http://hyper.ahajournals.org//subscriptions/