* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Fundamentals Of Organic Chemistry
Survey
Document related concepts
Asymmetric induction wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Elias James Corey wikipedia , lookup
Aromaticity wikipedia , lookup
Stille reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Aromatization wikipedia , lookup
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Macrocyclic stereocontrol wikipedia , lookup
Homoaromaticity wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Transcript
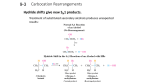
Fundamentals Of Organic Chemistry Part-4 (d) Dehydration removal of water In process of dehydration inter mediate carbocation formation takes place so that always remember reactivity of alcohols towards dehydration due to intermediate stability is 3°>2°>1°>CH3OH more the stability of intermediate higher is the reactivity of the initial compound (vi) Molecular rearrangement reaction For the stability of the molecule molecular rearrangement occurs in different chemical reactions alkyl shift and hydride shifts are most common rearrangement. Takes place in carbocation which is known as Wagner's rearrangement. Reactions involving the change in the carbon skeleton through the rearrangement of the carbonium intermediate by alkyl and hydride shift collectively known as Wagner–Meerwein rearrangement. When neopentyl bromide is hydrolysed under S N1 (due to bulky alkyl group) condition it is found that instead of the expected neopentyl alcohol (Me3CCH2OH), due to alkyl shift, 2-methylbutan-2-ol and 2-methyl but-2-ene are formed. Similarly, neopentyl alcohol on dehydration by the formation of carbocation gives 2-methylbut-2-ene and 2-methyl-but-1-ene here alkyl shifts occurs in the carbocation. In above cases, changes in the carbon skeleton are observed for stability. Such rearrangement was first observed in many bicyclic compounds. Mechanism of the dehydration of alcohol is discussed below, and also in elimination reactions, later in this book. Mechanism Protonation of the hydroxyl group of the alcohol followed by the loss of water molecule to give a 1° carbocation. The 1° carbocation undergo rearrangement to form more stable 3° carbocation by 1, 2-alkyl shift before the product is formed. Finally occurs and products are formed according to saytzeff's rule. 3, 3-dimethyl-2-bromo butane (neopentyl type) undergoes SN1 hydrolysis with rearrangement due to stability factor. While its phenyl analogue gives product without rearrangement because it is already stable by conjugation. The benzilic cation is stabilised by the phenyl group through delocalization and so their is no rearrangement. Rearrangement in the alicyclic system may involve migration of ring methylene group resulting in the ring expansion and contraction. Some simpler examples are: Ring expansion takes place due to molecular strain. Ring expansion takes place because small rings are under strain so they tends to convert into stable ring. Stability of these rings are The aryl group migrates faster than alkyl group. Normally electron-releasing group in the aryl group increases the attitude of migration while electron-attracting nature decreases the rate of migration. So that elimination is much faster in neophenyl bromide than in neopentyl bromide.