* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 15
Woodward–Hoffmann rules wikipedia , lookup
Marcus theory wikipedia , lookup
Hydroformylation wikipedia , lookup
George S. Hammond wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Homoaromaticity wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aromaticity wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
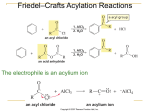
Ch 15 Reactions of Aromatic Compounds Intro • Aromatic hydrocarbons are known generally as arenes. • An Aryl group is formed by the removal of a hydrogen, and is symbolized with Ar• We have already talked about how stable the double bonds are in arenes due to conjugation. • They resist typical addition reactions Intro • We have also said that the pi electrons form clouds above and below the ring • This makes nucleophilic attack very unlikely as well • The most characteristic reactions of arenes are a new type of reaction called Electrophilic Aromatic Substitution, (EAS) EAS • Generic reaction: • The electrophiles in these reactions are either a positive ion (E+) or some other electron deficient species with a large partial positive charge EAS • Mechanism: • Aromaticity– Broken in 1st step- reactions usually require high temps – Regained in 2nd step- reason for substitution instead of addition • Arenium Ion- somewhat stabilized by resonance; delocalized positive charge Arenium ion • Substituents already on the ring can effect the stability of the arenium ion, and therefore effect the reaction. • Substituents on the ring are divided into 2 classification • The first deals with how the substituents effect the reactivity of the ring • The second deals with how the substituents effect the orientation of the incoming electrophile Reactivity of the Ring • Those substituents whose presence make the ring more reactive than benzene are called activating groups, or Activators • Those that make the ring less reactive than benzene are called De-activating groups, or Deactivators Reactivity of the Ring • If we look at the energy diagram we see that the 1st step is the RDS and that the Arenium ion is a true intermediate, not a transition state. • By increasing the stability of the arenium ion, we would be lowering it energy, therefore, lowering the activation energy for the RDS, making the reaction occur faster. Reactivity of the Ring • This is how Activating groups work • They help to stabilize the Arenium Ion • How can the Arenium Ion be stabilized? Reactivity of the Ring • Activating groups stabilize the arenium ion by donating electrons into the cation. • Strong Activators • Moderate Activators • Weak Activators Reactivity of the Ring • Deactivating groups have the opposite effect • They decrease the stability of the arenium ion, thereby increasing it energy, which increases the activation energy for the reaction, making the reaction occur slower. • Note: Notice I said slower! The reaction will still occur, just at a slower relative rate compared to benzene. Reactivity of the Ring • Don’t think that just because a ring has a deactivating group, that it won’t undergo a reaction. • For most reactions, it will still react just with a slower relative rate. • How would a group deactivate a ring? Reactivity of the Ring • Deactivators destabilize the arenium ion by withdrawing electrons from the cation ion. • Strong Deactivators • Moderate Deactivators • Weak Deactivators Orientation • The second classifications groups can be divided into deals with the way they influence the orientation of the incoming electrophile • Some substituents tend to direct the electrophiles to the ortho and para positions relative to themselves; while others tend to direct the electrophile to the meta positions. Orientation • To understand how these groups “direct” the incoming electrophile, we again look at the arenium ion. • More specifically, we look at the resonance structures of the arenium ion once the electrophile adds to the ring. Orientation • Notice with both the ortho and para positioning of the electrophile, the carbocation can be shown on the carbon directly bonded to the substituent • With the meta positioning of the electrophile, the carbocation never reaches the carbon attached to the substituent. Orientation • Activators are electron donors, so their stabilizing effects would be greater with the carbocation directly bonded to them. • Thus, all activators are ortho/para directors • By contrast, deactivator are electron withdrawing groups, so they want to avoid being directly bonded to the carbocation • Thus, strong and moderate deactivators are meta directors Exception • Of course there is an exception! • Halogens are weakly deactivating due to their electronegativity, but ortho/para directors due to an additional resonance structure. Types of EAS Reactions • All EAS reactions occur through the same, basic mechanism. • The only difference is the way the electrophile is created/introduced • Examples: Halogenation of Benzene • Benzene does not react with chlorine or bromine unless a lewis acid catalyst is present • Examples: • Mechanism: Nitration of Benzene • Examples: • Mechanism: Sulfonation of Benzene • General reaction: • This reaction is an equilibrium • We can influence the equilibrium by the methods we use • By using conc. H2SO4 or fuming H2SO4 (contains added SO3), the equilibrium is pushed to the right, increasing the amount of sulfonated benzene. Sulfonation of Benzene • By using dilute acid and steam, we can push the equilibrium to the left, removing the sulfonyl group. • The steam helps remove the volatile aromatic compound as it forms • Mechanism: Sulfonation of Benzene • The control of this equilibrium is fairly simple and is therefore very useful! • Often, we may introduce a sulfonic acid group to influence other reactions, then remove it later. • Example: Friedal-Crafts Alkylation • Discovered in 1877 as a new method of making alkyl benzenes, (Ar—R) • General Reaction: • Mechanism: Friedal-Crafts Alkylation • Friedel-Craft alkylations are not restricted to alkyl halides and aluminum chloride • Many other pairs of reagents that form a carbocation or species like carbocations, can be used. • Examples: • There are several important limitations of FC reactions. • We will discuss these after the FC acylations Friedal-Crafts Acylation • The acyl group is basically half a ketone, it’s an alkyl bonded to a carbonyl • A reaction which introduces an acyl group is an acylation • There are two common acyl groups: • The F.C. Acylation reaction is an effective means of introducing an acyl group onto an aromatic ring Friedal-Crafts Acylation • FC Acylations are often carried out using an aromatic ring and an acyl chloride in the presence of a lewis acid • Examples • The acyl chloride, aka acid chlorides, can be prepared by adding thionyl chloride, SOCl2 or phosphorus pentachloride, PCl5, to carboxylic acids • Examples: Friedal-Crafts Acylation • FC acylations can also be carried out using carboxylic acid anhydrides as the electrophile • Example • In most FC acylations, the electrophile is the acylium ion • Mechanism: Limitations to FC Reactions • As we said, there are several restrictions that limit the usefulness of FC alkylations and acylations – 1) When the carbocation formed from the alkyl halide, alkene, or alcohol can rearrange to a more stable carbocation, it usually does so and the major product obtained from the reaction is usually the one from the more stable carbocation. – Example: Limitations to FC Reactions • 2) Friedel-Crafts reactions usually give poor yields when powerful electron withdrawing groups are present on the aromatic ring or when the ring bears an –NH2, -NHR, or –NR2 group. • This includes all meta directing groups plus the amine groups • The deactivated rings are too electron deficient to undergo a FC reaction Limitations to FC Reactions • As for the amines, they become deactivating groups due to their reaction with the lewis acid. • Example Limitations to FC Reactions • 3) Aryl and vinylic halides cannot be used as the halide component because they do not form carbocations • Examples • 4) Polyalkylations often occur – When putting activating groups on, in general, such as alkyl groups, the product after the first addition is more reactive than the starting material – Example – Polyacylations do not occur since the acyl group is a deactivating group Synthetic Applications • Applications of the Friedel-Crafts Acylation reaction followed by the Clemmensen Reduction • Acylations have the advantage of not rearranging due to the resonance stabilized acylium ion. • Since they do not rearrange, FC Acylations followed by reductions provide a better way for producing unbranched alkyl benzenes. • Examples Clemmenson Reduction • A Clemmenson reduction is used to reduce the ketone adjacent to the aromatic ring down to the methylene group. • Example • The clemmenson reduction only reduces the carbonyl adjacent to the aromatic ring! • Example Reactions of the Side Chain of Alkyl Benzenes • Compounds that have both aromatic and aliphatic groups are also called arenes • Toluene, ethyl benzene, and isopropylbenzene are alkylbenzenes • Phenylethene, commonly known as styrene, is an example of an alkenylbenzene • The aliphatic portion of these compounds is commonly called the side chain Benzylic Radicals and Cations • The carbon directly attached to the aromatic ring in a alkyl benzene is the benzylic carbon • Hydrogen abstraction from this carbon creates the benzyl radical • Example • Departure of a leaving group from the benzylic position produces a benzylic carbocation • example Benzylic Radicals and Cations • Both radicals and cations are conjugated unsaturated systems and both are unusually stable • They have approximately the same stability as the allylic radical and cation • This can be show with resonance • example Halogenation of the Side Chain • Reaction via benzylic Radicals • Earlier we saw we could add bromine and chlorine to the actual ring with a lewis acid present • This is done by creating an electrophile out of the bromine and/or chlorine • We can add a bromine and/or chlorine to a benzylic carbon by promoting radical conditions and not using a lewis acid catalyst • Examples: • Mechanism: Halogenation of the Side Chain • Because of the stability of the benzylic radical, it forms faster than other radicals, therefore, halogenation at the benzylic site is always the major product • Example: Alkenyl Benzenes • Alkenyl benzenes that have their double bonds conjugated with the benzene ring are more stable than those that do not: • This is proven by the acid catalyzed dehydration reaction which is known to give the most stable alkene: Alkenyl Benzenes • Although the conjugated double bond is more stable, the same reactions are still seen: – HBr addition in presence of peroxides: – HBr addition with no peroxides: Oxidation of the Side Chain • Strong oxidizing agents oxidize the alkyl groups of alkyl benzenes to benzoic acid using potassium permanganate: • Example • The first step in this process is the abstraction of a benzylic hydrogen! (meaning there has to be one there!!) Oxidation of the Side Chain • In order for the oxidation to take place, there must be a benzylic hydrogen or the benzylic carbon must be unsaturated • Examples: Oxidation of the Benzene Ring • Conversely, the benzene ring of alkylbenzenes can be oxidized to the carboxylic acid by using ozone followed by peroxide • General reaction: • Example: • Note: Must watch for other functional groups Synthetic Applications • The substitution reaction of aromatic rings and the reactions of side chains of alkyl and alkenyl benzenes, when taken together, offer us a powerful set of reactions for organic synthesis with great regio-control • Part of the skill is using these reactions is deciding the order in which reactions should be carried out Examples • Synthesize o-bromonitrobenzene from benzene. • Synthesize p-nitrobenzoic acid from benzene Use of Protecting/Blocking Groups • Very powerful activating groups such as amino groups and hydroxyl groups cause the benzene ring to be so reactive, that multiple and/or undesirable reactions may take place • These groups may also react with some of the reagents, such as HNO3 and H2SO4 Use of Protecting/Blocking Groups • Amino groups must be protected by either adding acetyl chloride or acetic anhydride to convert the amino group to an acetamido group • The acetamido group is only mildly activating and can easily be removed with dilute acid • examples Use of Protecting/Blocking Groups • Groups such as the –SO3H, which are easy to put on and take off, are also used as blocking groups • For example, say we wanted to make o-nitroaniline: • Because of the size of the acetamido group, the ortho sites are sterically hindered, so if we add the nitro at this point, the major product would be para. Use of Protecting/Blocking Groups • Instead, we add a sulfuric acid group, which goes para, then the nitro which can only go ortho to the acetamido group • The sulfuric acid group can the be removed along with the acetyl chloride group at the same time. Orientation in Disubstituted Benzenes • When two different groups are present on a benzene ring, the more powerful activating group generally determines the outcome of the reaction • Example • Because all ortho/para directors are more activating than meta directors, the ortho/para director determines the orientation of the incoming group • Example: Orientation in Disubstituted Benzenes • Sterics also play an important role • Substitution does not occur between meta substituents if another position is open and directed • example Allylic and Benzylic Halides in Nucleophilic Substitution Reactions • Allylic and benzylc halides are classified in the same way as other halides, just add the allylic or benzylic • Tertiary allylic and benzylic halides still only undergo Sn1 reactions due to the steric hinderance. • The difference is with the primary allylic and benzylic halides. • Because they would form a very stabilized carboncation, primary allylic and benzylic halides may undergo Sn1 reactions The Birch Reduction • Benzene can be reduced to 1,4cyclohexadiene by using an alkali metal (Na, Li, or K) in a mixture of liquid ammonia and an alcohol • Example: • This reaction is the only example we will see that combines ionic and radical steps in the mechanism! • Mechanism: The Birch Reduction • Substituent groups on the ring can affect the reaction as well • One very important example of the Birch reduction includes: