* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download frontal functions, connectivity and neural efficiency underpinning
Cortical cooling wikipedia , lookup
Environmental enrichment wikipedia , lookup
Optogenetics wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Human multitasking wikipedia , lookup
Neuroplasticity wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Functional magnetic resonance imaging wikipedia , lookup
History of neuroimaging wikipedia , lookup
Limbic system wikipedia , lookup
Human brain wikipedia , lookup
Source amnesia wikipedia , lookup
Embodied cognitive science wikipedia , lookup
Embodied language processing wikipedia , lookup
Dual consciousness wikipedia , lookup
Neuropsychology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neurolinguistics wikipedia , lookup
Cognitive flexibility wikipedia , lookup
Lateralization of brain function wikipedia , lookup
Time perception wikipedia , lookup
Neuroesthetics wikipedia , lookup
Impact of health on intelligence wikipedia , lookup
Metastability in the brain wikipedia , lookup
Mental chronometry wikipedia , lookup
Neuroeconomics wikipedia , lookup
Affective neuroscience wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Neurophilosophy wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Aging brain wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Executive functions wikipedia , lookup
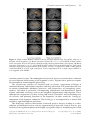
Contemporary Hypnosis 15 Contemp. Hypnosis 23(1): 15–32 (2006) Published online in Wiley InterScience (www.interscience.wiley.com) DOI: 10.1002/ch.35 FRONTAL FUNCTIONS, CONNECTIVITY AND NEURAL EFFICIENCY UNDERPINNING HYPNOSIS AND HYPNOTIC SUSCEPTIBILITY John H. Gruzelier Department of Psychology, Goldsmiths College, University of London, UK Abstract An update is provided of an earlier review (Gruzelier, 1998) of the range of evidence for neurophysiological changes in frontal and lateralized functions with hypnosis, changes which have differentiated high from low hypnotically susceptible subjects, and which led to a working model and neuropsychological translation of the hypnotic induction process. New evidence is outlined from an fMRI/EEG study. This study also disclosed the importance of neural efficiency in left lateral frontal and anterior cingulate structures, and their connectivity, for distinguishing high from low hypnotic susceptibility both in hypnosis and in the everyday state. This amplifies earlier constructs such as cognitive flexibility. Though the focus will be largely on the alteration of connections with the anterior brain and its corresponding alterations of function, interhemispheric, posterior and subcortical connectivity is also considered. The practical implications for the interaction between the hypnotherapist and subject are considered, including stage hypnosis. Copyright © 2006 British Society of Experimental & Clinical Hypnosis. Published by John Wiley & Sons, Ltd. Key words: EEG, fMRI, hypnosis, hypnotic susceptibility, neurophysiology, stage hypnosis Neurocognitive changes with hypnosis Selective inhibition, dissociation and disconnection Since the time of Janet dissociation has been historically the dominating cognitive theory of hypnosis (Hilgard, 1965; Bowers, 1992). Now a range of evidence is showing that following instructions of hypnosis a different and unusual pattern of abilities and disabilities is brought into play in hypnotizable subjects compared with the pre-hypnosis state. These involve dissociations between cognitive processes, and disconnections between brain regions together with selective inhibition and enhancement of abilities and processes (Gruzelier, 1998; 2004). The likening of hypnosis to an inhibitory process is enshrined in the term hypnosis, itself derived from Hypnos the god of sleep, and initially made popular by Pavlov’s inhibitory concepts. Early electrophysiological studies found no evidence of sleep per se (Crawford and Gruzelier, 1992), nevertheless frontal inhibition may represent one dynamic of frontal involvement in hypnosis (e.g. Gruzelier, 1990; 1998; Woody and Bowers, 1994; Woody and Sadler, 1998), and one which may be an alternative to disconnection, or be in parallel with it. The experimental evidence for changes in anterior brain activity will be considered in turn. Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 16 John Gruzelier Error detection and evaluation Neurophysiological fractionation of processes, to include possible inhibition, has been demonstrated between error detection processes and error evaluation processes and with the apparent inhibition or uncoupling of the latter, both of which are processes emanating from the anterior cingulate. These processes were measured with event-related potentials (ERPs) in a Stroop-like conflict task (Kaiser, Barker, Haenschel, Baldeweg and Gruzelier, 1997). The ERPs disclosed a dissociation not evident behaviourally. In the highly hypnotizable participants error detection rates decreased and reaction times (RTs) were prolonged following instructions of hypnosis, as has been reported by others (Nordby Hugdahl, Jasiukaitis and Spiegel, 1999; Jamieson and Sheehan, 2004). Coincidentally with the behavioural impairment error detection waves were unaltered, implying that mistakes in performance were being detected but went uncorrected. But an electrophysiological change did accompany the impaired performance, and this involved the ensuing positive wave that follows error-related negativity. This ‘error-related positivity’, which is associated with error evaluation processes, was abolished. In contrast to the apparent neurocognitive dissociation in the hypnotizable participants, the low hypnotizable group, acting as a control for the effect of hypnosis, showed no falloff in performance behaviourally following instructions of hypnosis, and no change in their event-related potentials, which contained both error detection and error evaluation waves. Using ERP source localization procedures, the processes of error detection and evaluation, as reflected in the error-related negativity and positivity, have indeed localized to the anterior cingulate. In sum a neurocognitive dissociation was demonstrated following hypnosis. For while hypnosis did not interfere with the detection of errors in performance, hypnosis did interfere with the deeper processing of the errors, theorized to give rise to a falloff in behavioural performance shown by the slow RTs. This dissociation may reflect either inhibition/deactivation or disconnection. The psychological dissociation with hypnosis has features in common with the dissociation implicit in Hilgard’s demonstration of a hidden observer. Here pain analgesia is experienced, yet simultaneously the subject can rate the intensity of the administered pain. As an incidental finding, in the same experiment during hypnosis an alteration specific to the hypnotizable subjects was found in EEG alpha coherence in the left frontal lobe (Gruzelier, 1998). This will be seen to have parallels in the recent fMRI/EEG study. Pain and hypnotic analgesia The anterior cingulate has also been implicated in the underpinning of hypnosis-induced analgesia (Faymonville, Laureys, Degueldre, DelFiore, Luxen, Franck, Lamy and Maquet, 2000; Rainville, Duncan, Price, Carrie and Bushnell,, 1997; Rainville, Hofbauer and Paus, 1999; Derbyshire, Whalley, Stenger and Oakley, 2004), as we have demonstrated electrophysiologically (Croft, Williams, Haenschel and Gruzelier, 2002). Here ‘inhibition’ as an explanatory concept can be ruled out. In response to painful stimuli we examined fast frequency 40 Hz gamma oscillations in view of their putative role in underpinning conscious perception (e.g. Tallon-Baudrey and Bertrand, 1999). In keeping with this evidence the magnitude of frontal gamma oscillations in the pre-hypnosis state was found positively correlated with the intensity of the affective response to pain. However, following instructions of hypnosis, while the relation was retained in those with low hypnotizability, the relation no longer held in those with high hypnotizability. In other words outside of hypnosis the greater the distress experienced by the pain stimuli, the higher the amplitude of the gamma oscillations, whereas during hypnosis distress was unrelated to the magnitude of the gamma oscillations. Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch Frontal connectivity and hypnosis 17 Application of low resolution source localization methodology (LORETA) confirmed that the generation of the gamma oscillations arose from the midline anterior cingulate, in keeping with the extensive literature on the subjective distress of pain and this frontal structure. Of theoretical importance was the finding that the magnitude of the gamma oscillations was unchanged, indicating no change in arousability/activation per se. The results support a selective dissociation with hypnosis of frontal and anterior cingulate processes from somatosensory cortices, without alteration in anterior activation levels; in other words with no evidence of inhibition. Voluntary auditory attention In an auditory attention task, with recording of frontal and parietal ERPs, an accumulation of anterior inhibitory processes was disclosed with the course of hypnosis in hypnotizable subjects (Gruzelier, Gray and Horn, 2002). Specifically, from the prehypnosis baseline to early and later stages, over 40 minutes of hypnotic induction, the hypnotizable participants showed a progressive reduction of attention related negativity (N100) in frontal electrodes. These dynamics were not paralleled by changes in subsequent cognitive memory-related components (P300), nor were they found in parietal recordings. This provided further evidence of regional changes and/or dissociated changes in hypnotizable subjects. We note that bilateral attenuation of the N100 difference wave is characteristic of frontally lesioned patients, whether or not the lesion is lateralized. Interestingly in subjects with low hypnotic susceptibility the N120 was negligible in their pre-hypnosis baseline, and became progressively larger with hypnosis. This was the opposite result to the one seen in hypnotizable subjects. The fact that both low and high hypnotizable groups undergo changes with hypnosis, but often in opposite directions, was disclosed in a previous study, now considered. Automatic auditory attention The concept of inhibition was introduced in our first study of hypnosis published two decades ago (Gruzelier and Brow, 1985). This involved electrophysiological recording of orienting and habituation processes to novel tones interspersed with the induction of hypnosis. Orienting responses were recorded with electrodermal activity, a pure measure of sympathetic autonomic responsiveness. Highly susceptible subjects showed a reduction in responses with hypnosis when compared with several control conditions, whereas subjects with low susceptibility showed increased responding, the opposite effect. At the same time both groups shared evidence from other autonomic parameters of attentional engagement. In the subjects with low hypnotizability reverse effects were found with more orienting responses and slower habituation, a pattern which is in keeping with enhanced sustained attention as may occur with apprehension. The facilitation of habituation with hypnosis in hypnotizable subjects was then replicated in an experiment designed to compare hypnosis with simulating hypnosis in subjects with medium/high hypnotizability (Gruzelier, Allison and Conway, 1988). Critical central influences on autonomic orienting responses include frontal modulatory connections of the orbitofrontal and dorsolateral cortices with the limbic system, particularly the amygdala and hippocampus. The amygdala has been shown to exert mainly excitatory influences on orienting activity whereas the inhibitory action of the hippocampus facilitates the habituation (inhibition) of the orienting response with stimulus repetition (e.g. Gruzelier and Venables, 1972; Pribram and McGuinness, 1975; Gray, 1982). Accordingly the highly hypnotizable group showed evidence in line with reduced orbitofrontal-amygdaloid excitatory influences on responding and increased dorsolateralCopyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 18 John Gruzelier hippocampal inhibitory influences. The influence of hypnosis on electrodermal orienting and habituation was compatible with the neuroanatomical evidence of De Benedittis and Sironi (1988) arising from recordings of intracranial electrical activity obtained during hypnosis. They found that hypnosis involved functional inhibition of the amygdala and activation of the hippocampus. We also found evidence of shifts in hemispheric influences as a result of hypnosis in the hypnotizable subjects now considered. Hemispheric asymmetry as a dynamic in dissociation and disconnection Electrodermal orienting response asymmetries In the orienting response study above, electrodermal activity was recorded bilaterally to investigate hemispheric influences (Gruzelier, Brow, Perry, Rhonder and Thomas, 1984). Despite the attenuation in responding occurring with hypnosis, a reversal in asymmetry in orienting response amplitudes could be detected when compared with the neutral condition. With hypnosis right hemispheric frontolimbic influences predominated in highly susceptible subjects. Outside of hypnosis hypnotizable subjects were also asymmetric, in fact in the course of stimulus repetition they showed a shift from an initial right-sided preference, in line with the right hemisphere’s role in global orienting, to a left hemispheric preference, in line with left-sided involvement in the local orienting process. This dynamic was compatible with the putative flexibility of hypnotizable subjects in their neurocognitive processing abilities, as outlined below. In those with low susceptibility there was no reliable asymmetry in either condition. In other words hypnosis produced a shift in the activational balance of fronto-limbic influences to favour the right hemisphere. Ideational fluency The first clear neuropsychological finding of left frontal involvement in hypnosis involved delineation of dissociations between three neuropsychological tests of ideational fluency. These were selected to differentiate between anterior left and right hemispheric functions and also within the left hemisphere to delineate between anterior dorsolateral and temporal functions (Gruzelier and Warren, 1993). The results disclosed an imbalance in dorsolateral prefrontal functions, an imbalance that disadvantaged prefrontal left hemisphere processes (indexed by word fluency to letter designated categories), and favoured anterior right hemisphere processes (indexed by fluency for designs). At the same time left temporal functions (indexed by fluency for words belonging to semantic categories) remained activated. The latter result would follow the necessary left temporal word comprehension abilities required in listening to the verbal induction; though with practice these might be given over to rudimentary right hemispheric comprehension abilities (Gruzelier, 1998). This differential pattern between the two types of verbal fluency generation was replicated by Kallio et al. (2001). They also found that the reduction in fluency correlated positively with hypnotic susceptibility, and also with interference on the Stroop conflict task, a task also shown to involve anterior functions to include the anterior cingulate (Botvinik, Nystrom, Fissell, Carter and Cohen, 1999). These unique patterns of neuropsychological abilities and disabilities between cognitive abilities could not be anticipated by social factors including expectation or task demands, explanations favoured by some theorists, nor were they part and parcel of normal functioning, all of which rules out an explanation based on expectancy. Expectancy theory could not have predicted the dissociation between the three tests Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch Frontal connectivity and hypnosis 19 (Gruzelier, 2000a) and the focal inhibition/deactivation of the left prefrontal ideational process. Haptic discriminations Lateral functional shifts with hypnosis have been demonstrated in somatosensory processing in a total of three investigations. Here reductions in left hemispheric haptic processing times coincided with enhancements in right hemispheric processing (Gruzelier et al., 1984; Cikurel and Gruzelier, 1990), and in the first experiment the degree of reduction in the left hemispheric processing time correlated with the depth of hypnosis. It was also the case that the hypnotizable subjects had superior left than right hemispheric discriminations in the prehypnosis state. One of the follow-up experiments (Cikurel and Gruzelier, 1990) contained the activealert hypnosis procedure of Banyai and Hilgard (1976) with participants pedalling a stationary bicycle with instructions of mental invigoration. The demonstration of the hemispheric shift in activation while pedalling precluded an explanation of the lateral shift based on a nonspecific factor such as relaxation. Deep relaxation measured by sensory reduction in a floatation tank while producing right hemispheric haptic enhancement, was without the simultaneous reductions (inhibition) in left hemispheric haptic abilities (Raab and Gruzelier, 1994), found with hypnosis. Thus relaxation is an important component but cannot account for the left anterior inhibitory effects of hypnosis. Brightness discrimination Hemispheric asymmetry of function in visual processing in hypnosis was examined using a standard divided visual-field apparatus in which flashes of light of varying brightness were presented in the peripheral visual fields (McCormack and Gruzelier, 1993). Following instructions of hypnosis an enhancement of right hemispheric perceptual sensitivity for brightness discriminations (the d prime psychophysical metric derived from signal detection theory) was found in highly susceptible subjects. On the other hand left hemisphere sensitivity remained unchanged, and therefore was seen to be independent/dissociated from the right hemisphere. Cognitive confounds were excluded because there was no corresponding hemispheric dissociation in the psychophysical index of cognitive influences on perception (beta). Interestingly comparisons of the highly hypnotizable subjects with those with medium levels of susceptibility indicated in the latter an increase in perceptual sensitivity which was bilateral. Bilateral enhancement would be consistent with a less focal enhancement of posterior processes with hypnosis. This result was also found in the previous haptic sorting study in participants with moderate levels of hypnotic susceptibility (Cikurel and Gruzelier, 1990). Tone probe ERPs Cortical evoked potentials (ERPs) were recorded to tone probes from electrodes placed over the anterior temporal lobes bilaterally at T3 (left) and T4 (right). This probe strategy enables cortical activation patterns to be assessed during concurrent tasks. The tone probes were presented simultaneously either with a hypnotic induction or a story read by the hypnotist, or were presented in a neutral baseline condition. The temporal electrode placements were compared with central electrode derivations (C3,4), and the two tasks were referred to the baseline where there was no cognitive task (Jutai, Gruzelier, Golds and Thomas, 1993). In participants with medium/high susceptibility the N100 attentional wave to the tones disclosed greater right temporal responsivity (T4) following Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 20 John Gruzelier the induction of hypnosis, whereas the expected left temporal advantage (T3) was shown when listening to the control story. In contrast participants with low hypnotizability showed the left-sided preference to both the story and hypnosis. The results demonstrated a lateral advantage in favour of right anterior temporal lobe activity specific to the more hypnotizable participants with hypnosis, suggestive of a right hemispheric processing of the hypnotist’s message, relying on the more rudimentary right hemispheric comprehension abilities. The group with low hypnotizability exhibited left anterior temporal activation, which did not distinguish between the hypnosis and story conditions, and was congruent with left hemispheric preference for verbal comprehension. Neural efficiency and individual differences in hypnotic susceptibility The thesis is developed elsewhere (Gruzelier, 2002a) that hypnotizability has many advantages, aside from susceptibility to hypnosis, and these stem from the putative underpinnings of neurophysiological and cognitive flexibility/efficiency. Evans (1991) has reviewed two decades of his research on the theme of flexibility. He has related hypnotizability, inter alia, with the facility for random number generation, and the flexible control of sleep (Evans and Graham, 1980). He reported that the ability to respond to suggestion in sleep in the REM phase correlated positively both with hypnotizability and with the facility for falling asleep in the laboratory; a phenomenon which signifies the ability for dissociative control outside of awareness and volition. Crawford (1989) and Crawford and Gruzelier (1992) used cognitive flexibility as an explanatory construct when reviewing cognitive and neurophysiological findings that have differentiated high from low hypnotic susceptibility. These included task-related hemispheric specificity found only in highly susceptible subjects independent of hypnosis, along with functional neuropsychophysiological changes as a result of hypnotic instructions. Similarly the ability to prime wider networks of association between cortical representational networks has been theorized to be associated with both hypnotizability and hypnosis (Shames and Bowers, 1992). The cognitive, affective and neurophysiological flexibility of the hypnotizable participant includes superior abilities in absorption, creativity, dissociation, attention and vividness of imagery; these are all well known correlates of hypnotizability (Gruzelier, 2002a, 2006). Recent evidence includes adaptive advantages for protection against cardiac hazard (Santarcangelo and Sebastiani, 2004). The experiments that have been outlined in the previous sections disclosed several examples of differences between hypnotizability groups in control conditions without hypnosis. Highly hypnotizable participants had superior left hemispheric haptic sorting ability (Gruzelier et al., 1984) and word fluency (Gruzelier and Warren, 1993), and exhibited hemispheric cognitive congruency in an apparent shift from global (right hemisphere) to local (left hemisphere) orienting processes (Gruzelier et al., 1984). Perhaps most striking of all was the difference in the ERP N100 attentional component across the frontal chain in the auditory detection task (Gruzelier et al., 2002). This component was augmented in highly hypnotizable subjects, reflecting the engagement of focussed attention processes, whereas it was virtually absent in those with low hypnotizability, as would occur with the failure to engage frontal attentional circuits which may follow distraction. In line with the frontal model of hypnosis the N100 attention wave was progressively attenuated in hypnotizable subjects with the course of hypnosis, and as would occur with disengagement of frontal processes. In those with low hypnotizability Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch Frontal connectivity and hypnosis 21 the N100 attention wave progressively increased, as would occur with progressive engagement of attention. A similar increase in responsiveness was also shown in the electrodermal orienting responses of those with low hypnotizability (Gruzelier and Brow, 1985), whereas responses were attenuated with hypnosis in hypnotizable subjects. These group characteristics led to predictions about BOLD measurements in fMRI now described. Conflict monitoring with fMRI and EEG But first to consider the dynamics of blood flow oxygenation and cognitive processing and their implications for individual differences. As will be seen this largely overlooked issue has somewhat discomforting implications for the field of brain localization with fMRI and PET. For essentially the more efficient the cognitive processing is, the less the oxygenation requirement. With learning activation decreases, so that the greater the learning and the higher the intelligence the greater the decrease in activation, especially in frontal brain regions associated with reasoning (Haier, Siegel, Maclachlan, Soderling, Lottenberg and Buchsbaum, 1992a; Haier, Siegel, Tang, Abel and Buchsbaum 1992b; Duncan, Seitz, Kolodny, Bor, Herzog, Ahmed, Newell and Emslie, 2000; Gray and Thompson, 2004; Neubauer, Grabner, Freudenthaler, Beckman, Guthke, 2004). In other words the more intelligent brain requires less metabolism and consequently is less likely to show fMRI activation. One implication for fMRI studies in general is that one cannot be certain that less intelligent/efficient brains will process in the same way as less intelligent/efficient brains so that regions utilized for processing in the former may go undetected. Notwithstanding, the clear implication here is that when considering the flexibility hypothesis, less activation will be predicted at baseline in participants with high hypnotizability than in those with low hypnotizability. We investigated conflict monitoring with a Stroop-like interference task while subjects were scanned with fMRI and while their EEG was recorded in a separate session (Egner, Jamieson and Gruzelier, 2005). In both sessions we successfully demonstrated the Stroop conflict effect on behavioural performance and found that in line with other evidence (Carter, MacDonald, Botvinick, Ross, Stenger, Noll and Cohen, 2000; MacDonald, Cohen, Stenger and Carter, 2000; Botvinik, Cohen and Carter, 2004) the conflict task involved the anterior cingulate (ACC), which increased in activation with increasing levels of conflict monitoring. At the same time (with high levels of conflict), a cognitive control centre in the left lateral frontal cortex (LPC) was engaged (MacDonald et al., 2000). There were two main fMRI hypnosis findings disclosed by a significant Group x Session interaction in the blood oxygenation ACC response. In hypnotizable subjects there was an increase in ACC activation from baseline to hypnosis, and this was to a higher level than found in subjects with low hypnotizability in whom there was a nonsignificant decrease in activation. There was also a weak tendency for those with low hypnotizability to have higher activation at baseline (p < 0.13). In other words the highly susceptible subjects were compromised by hypnosis, whereas those with low hypnotizability tended to improve, perhaps due to practice. These differential changes have a counterpart with our auditory discrimination ERP study (Gruzelier et al., 2002). It was also the case that the ACC compromise with hypnosis was not paralleled by compromise in the LFC (left inferior frontal gyrus) region that is associated with cognitive control and is activated significantly more in the higher difficulty level conditions. In other words there was no commensurate increase in blood oxygenation in the hypnotizable subjects in the LFC as had occurred in the ACC, a result representing an uncoupling or dissociation between the ACC and LFC with hypnosis. This interpretation was Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 22 John Gruzelier borne out by EEG recordings during the same task in a separate session. EEG coherence measures indicated that following instructions of hypnosis, what characterized the highly susceptible subjects was a reduction in connectivity between the anterior cingulate and the left dorsolateral prefrontal cortex (F3), as reflected in the gamma rhythm coherence, with the converse effect in those with low susceptibility. There was no such interaction between group and session in the corresponding right homolateral site (F4). This evidence assists in clarifying the nature of the alteration in frontal functions with hypnosis. The left inferior frontal locus that was activated in this investigation has been implicated in contention scheduling, and contextual control processes including associating external cues with appropriate actions (Passingham, Toni and Rushworth, 2000; Shallice, 2002; Koechlin, Ody and Kouneiher, 2003). This underscores the disruption of executive functions with hypnosis through disconnectivity. In a separate analysis, as presented at the British Association meeting, baseline differences between the groups emerged clearly, as shown in Figure 1. Blood oxygenation level-dependent (BOLD) responses to different levels of response conflict were assessed by random effect analyses comparing event-related activation in moderate versus low conflict trials (moderate conflict contrast), and in high versus low conflict trials (high conflict contrast), excluding error trials. Based on previous studies, the analyses were carried out for a priori regions of interest covering the ACC (Brodmann areas 24 and 32) and the left dorsolateral prefrontal cortex (Brodmann area 9). Following the report of Macdonald et al. (2000) moderate response conflict conditions activated cingulate and medial frontal gyri while the high response conflict contrast resulted in cingulate activation, and additionally activation in left superior, middle and inferior frontal gyri. These contrasts were analysed in the hypnotic groups separately. Clear differences in conflictrelated attentional processing emerged. At baseline, in the low susceptibility group both moderate and high conflict elicited substantial activation in both regions, whereas in highly susceptible participants the moderate conflict contrast did not result in any significant activation, and the high conflict contrast only produced limited cingulate activation. As above with hypnosis these patterns of conflict-related processing were reversed. Thus, without group differences in behavioural performance levels, highly susceptible participants were characterized by a higher efficiency of executive attention than participants with low susceptibility at baseline, and by relatively impaired executive function in the hypnotic state. Neuropsychological translation of the classical induction of hypnosis The earlier findings with hypnotic relaxation led to a neuropsychological translation of the nature of hypnosis as induced by the conventional procedure aimed at producing a state of relaxation (Gruzelier, 1988, 1990, 1998). It is important to note that for the main part in our studies we have deliberately avoided active challenges, which must provide an added level of complexity in elucidating fundamental processes in hypnosis, let alone the stunts involved in hypnosis for entertainment. Accordingly our hypnotic relaxation may be thought of as akin to what has been termed ‘neutral hypnosis’. As will be seen, anterior brain functions represent a cardinal region in the induction of the hypnotic process, and in accounting for the character of the hypnotic susceptibility trait. A three-stage working model of the induction process was evolved: The first stage involves the traditional instructions to fixate on a small object and to listen to the hypnotist’s voice. Here was posited an attentional network including thalamocortical systems and parietofrontal connections to engage a left anterior focussed Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch Frontal connectivity and hypnosis 23 Figure 1. High conflict BOLD responses in low and high hypnotically susceptible subjects, at baseline and in hypnosis. (A) Brain activation (Talairach x = 6, z = 35) in ROIs in high conflict trials at baseline for low (left panel) and high (right panel) hypnotizability participants. (B) Brain activation (Talairach x = 6, z = 35) in ROIs in high conflict trials in hypnosis for low (left panel) and high (right panel) hypnotizability participants. Activity is displayed at FDR = 0.05 with an extent threshold of at least eight contiguous voxels, superimposed on a single subject MNI T*1 scan supplied with SPM99. attention control system. This underpins the focussed, selective attention that is inherent in visual fixation and listening to the hypnotist’s voice. Together these processes require left hemispheric frontotemporal processing. The second stage replaces eye fixation with eye closure, suggestions of fatigue at continued fixation, and tiredness together with deep relaxation. It is posited that this sets in motion frontolimbic inhibitory processes with dissociative or uncoupling consequences, left-sided in particular, encompassing orbitofrontal and dorsolateral frontal regions and limbic structures such as the amygdala, hippocampus and cingulate. These underpin the suspension of reality testing and critical evaluation, and the handing over of executive and planning functions to the hypnotist; in other words the ‘letting go’ component of the hypnotic induction. This letting go is accompanied by a lateral shift towards a right hemispheric preference. The third stage involves instructions of relaxed, passive imagery leading to a redistribution of functional activity and an augmentation of posterior cortical activity, particularly of the right hemisphere in the highly susceptible subjects. Simplifying the verbal Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 24 John Gruzelier content of the induction message may also facilitate right hemispheric processing as does emphasizing past experience and emotion. The participants with low susceptibility in contrast fail to show engagement of left frontal attentional control mechanisms, or if there is focal attentional engagement, the subject with low susceptibility fails to undergo the ‘inhibitory’, letting go process. Accordingly this provides two reasons for ostensibly willing subjects not to undergo hypnosis. The retarded habituation of orienting responses to stimuli irrelevant to hypnosis is consistent with vigilant, broad attention or distractibility, and slow habituation occurs with anxiety (Gruzelier and Phelan, 1991). Letting go requires reassurance about the lack of unwanted consequences of hypnosis, such as a loss of control or failure to come out of hypnosis, which may preoccupy naïve subjects. We have theorized that the alleviation of these worries may account with practice for evidence of focusing of attention, and through practice with self-hypnosis allow benefits such as natural killer cell enhancement (Gruzelier, 2002b). In sum the ‘letting go’ stage, which is cardinal to hypnosis, is underpinned by the selective inhibition or disconnection of frontal functions from posterior and subcortical functions, leading to the giving over and the placing of the executive and planning functions under the hypnotist’s influence, to suspension of critical evaluation and reality testing, as well as to alterations in the control of the supervisory attentional system (Gruzelier, 1990, 1998; Crawford and Gruzelier, 1992; Woody and Bowers, 1994; Woody and Sadler, 1998). Oakley et al. (personal communication) investigating this three-stage temporal process for depth of hypnosis in an fMRI scanner confirm the impact of the ‘letting go’ stage for increasing the depth of hypnosis. A neurophysiological theory of altered top-down influences in stage hypnosis The author has proposed that frontal functions are fundamental to the stage hypnotist’s persuasion (Gruzelier, 2000b, 2004). The thesis began with a landmark neuropsychological case of an alteration in personality through a brain lesion. In 1848 Phineas Gage, a railway artisan working on the trans-Canadian railway, suffered a devastating insult to the head following a dynamite explosion which drove an iron bar through his cheek on a trajectory through the frontal cortex. After the accident the socially abiding and popular worker displayed extraverted, nefarious, impulsive and profane behaviour, making decisions against social convention and contrary to his best interest. Having undergone a change of personality and character he ended his life as a fairground sideshow (shades of the appeal of stage-hypnosis). Reconstruction with MRI from photographic images of the skull has confirmed that it was the ventromedial or orbital aspects of the frontal cortex that were damaged (Damasio, 1994). It should be emphasized that the damage was definitely not on a par with anything as pronounced as frontal lobotomy The Damasios undertook a programme of research on patients with damage to the ventromedial prefrontal cortex. They contrived a task that simulated real-life decision making while leaving intellectual functions unaffected. Patients were guided only by immediate prospects, and were oblivious to the consequences and positive or negative affective value of their future actions (Damasio, 1994; Bechara, Damasio, Damasio and Anderson, 1994; Bechara, Tranel, Damasio and Damasio 1996; Bechara, Damasio, Tranel and Damasio, 1997; Bechara, Damasio, Damasio and Lee, 1999; Bechara, Damasio and Damasio, 2000). Furthermore the patients continued to behave disadvantageously Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch Frontal connectivity and hypnosis 25 after being made aware of the consequences of their actions (Bechara et al. 1997; Bechara et al. 1999). From simultaneous recordings of autonomic electrodermal activity an absence of anticipatory responses was disclosed, and was interpreted as an unavailability of emotionally related knowledge with which to inform decision making in social situations. This led to a ‘somatic marker’ hypothesis, which, inter alia, proposed that a deficiency in affect and feeling in anticipation of action played a critical role in impaired decision making about the future actions of frontal patients (Damasio, 1994). It is the ventromedial prefrontal cortex which processes the association between behavioural outcome and the corresponding emotional outcome, an association contained in a dispositional rather than an explicit form. The emotional somatosensory image, via reactivation of a somatic memory, marks the potential outcome of actions as either positive or negative. In other words, it is not that the patient with medial frontal damage cannot experience and express emotion, as hypothesized to be the case in psychopathy or in the syndrome belle indifference: the patient does feel emotion, but there is a failure to adjust behaviour according to past experience, because the feelings based on past experience are no longer engaged. Accordingly there is a reliance on the immediate advantage as opposed to the future advantage, which can no longer be predicted accurately (Schoenbaum et al. 1998), and this appraisal is particularly faulty in unpredictable circumstances. Social behaviour is disrupted, and ‘previously well adapted individuals become unable to observe social conventions, and unable to decide advantageously on matters pertaining to their own lives’ (Bechara et al., 2000:295). This may be extrapolated to hypnosis, with its inherent selective suppression or disconnection of selected frontal functions, including orbito-frontal-amygdaloid connections, as shown in our electrodermal orienting response studies (Gruzelier and Brow, 1985; Gruzelier et al., 1988), and extending to the orbital frontal cortex (OFC) and its connections with the conflict monitoring capabilities of the anterior cingulate. Relevance to an understanding of stage hypnosis becomes apparent. On stage the hypnotically susceptible subject responds without embarrassment to the immediate contingencies, i.e. the instructions of the hypnotist to enact behaviour typically making the subject appear a fool, in order to provide entertainment for the audience. Furthermore the participant persists in doing so even though cognitively aware, just as is found in the OFC lesioned patient. Accordingly, what is missing in the stage hypnosis participant is the association, based on past experience, of the emotional consequences of the actions instructed by the hypnotist, such as emotional appraisal of likely humiliation. Behaviour is governed by the immediate context, i.e. the instructions of the hypnotist. Negative consequences can no longer be predicted. Thus neuropsychology of altered frontal functions, including selective disconnection, may offer an explanation for the attraction of hypnosis for entertainment. An explanation for why subjects allow themselves to be made a fool of, to suffer humiliation without embarrassment, often appearing to have undergone a personality change, and so to provide the necessary theatrical display to maintain the popularity of stage hypnosis with the general public, now chiming with the cultural appetite for ‘reality’ entertainment. Orbitofrontal cortex, right hemisphere and the therapeutic relationship Lateralized functional alterations leading to right hemispheric preferential activation were found to be an important dynamic of hypnosis, as outlined here, while for a review of earlier evidence see Crawford and Gruzelier (1992). The orbital and medial frontal cortex, theorised to play a role in stage hypnosis, has a special relationship with the right Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 26 John Gruzelier hemisphere. As Cavada and Schultz (2000:205) concluded in the foreword to an issue devoted to the orbital frontal cortex in the journal Cerebral Cortex, ‘The orbitofrontal cortex is involved in the critical human functions, such as social adjustment and the control of mood, drive and responsibility, traits that are crucial in defining the ‘personality’ of an individual.’ This region exerts the highest level of control over emotional behaviour, by virtue of having the only direct cortical connections with the amygdala, hypothalamus, and brain stem reticular activating system. This region, which is anatomically larger in the right hemisphere, takes executive control over the right hemisphere per se, and bilateral executive control of the limbic system and autonomic nervous system. The right hemisphere matures earlier than the left, and is dominant for the attachment process between mother and infant (Schore, 1994). Attachment involves the regulation of biological synchrony between people and transactions, which mediate the social construction of the brain for which the right hemisphere is dominant. The right hemisphere also has functional advantages for the control of attention and emotion, prosody and facial recognition, unconscious processes such as implicit memory and preattentive facial emotions, as well as the representation of somatic and visceral states and selfrelated material through its control over the limbic and autonomic nervous systems (e.g. Hugdahl, 1995; Pizzagali, Regard and Lehmann, 1999; Keenan, Wheeler, Gallup and Pasual-Leone, 2000). In therapy, just as in studies of the empathetic processes between the intuitively attuned mother and her infant, the affective synchrony is often nonverbal. Resonance is with affective bodily states, rather than being cognitive in nature (Schore, 1997). This spontaneous communication is mediated by the right-sided limbic system (Buck, 1994), which as shown in our limbic-mediated electrodermal recording becomes dominant in hypnosis (Gruzelier et al., 1984). The empirical results showing the shift of functional activity to advantage the right hemisphere with conventional hypnotic relaxation, support the well known clinical experiences and metaphors of access to right hemispheric processes with hypnosis (Pedersen, 1984)). Thalamo-cortical functions More than 70% of thalamocortical connections are with anterior cortex. This facilitates the frontal lobe’s executive functions in playing a moderating role throughout the cortical mantle as well as subcortically through the limbic structures and the brainstem. This gives rise to wider implications for frontal influences on behaviour in hypnosis. The release of functions from frontal inhibitory control may not only enhance posterior brain activity but also release subcortical activity. Thalamo-cortical connections, and especially prefrontal connections, are central to consciousness circuits (Zeman, 2002). These are directly involved in the alterations of consciousness experienced in hypnosis (Rainville et al. 2000) including alterations in arousal at either extreme, such as seizure and stupor (Kleinhauz and Behan, 1981). Thalamocortical loops that involve orbitofrontal-limbic circuits such as orbitofrontalhypothalamus-amygdala-brainstem reticular formation not only regulate internal arousal processes, but also appraise changes in the external environment by processing sensory information including the face and voice. This allows an integration of adaptive bodily responses with ongoing emotional and attentional states, and the regulation of interpersonal and social behaviour (Dolan, 1999; Critchley, Elliott, Mathias and Dolan, 2000). These circuits provide a mnemonic repository of visceral and somatic states and material related to self (Damasio, 1994). By a selective shutting down of customary everyday Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch Frontal connectivity and hypnosis 27 frontal executive and inhibitory functions, hypnosis provides access to past memories of cognitive, affective and somatic events (Damasio’s ‘somatic marker’). This may explain how somatic memory, including surgical operations in childhood that involve the alteration and reduction of consciousness with anaesthetic (Hilgard, Hilgard and Newman, 1961), and the reinstatement of epilepsy (Page and Handley, 1993), may arise with hypnosis. Such a shutting down mechanism therefore facilitates age regression. The association between hypnosis with an unpleasant childhood event has been a potent source of negative reactions involving revivification of emotionally laden and somatic events (or taking the form of retrograde amnesia), and alterations of arousal with autonomic signs. Sequelae have included chronic depression and PTSD symptoms (Gruzelier, 2000b, 2004); dissociative episodes following age regression to the time of WWII trauma (Kleinhauz, Dreyfuss, Behan, Goldberg and Azikri, 1979); surgical operations in childhood involving the alteration and reduction of consciousness with anaesthetic (Hilgard et al., 1961); the production of stupor (Kleinhauz and Behan, 1981); and the reinstatement of epilepsy (Page and Handley, 1993). Where there were multiple memory cues in this associative network of negative episodes, adverse effects may be compounded (Page and Handley, 1993). A cumulative memory effect was observed when an unpleasant experience in childhood with chemical anaesthesia, and the countdown while that anaesthetic took effect, were associated respectively with a change in arousal with hypnosis and the dehypnosis countdown (Hilgard et al., 1961). The reduction in frontal influences will also lead to cognitive confusion and distortions of body schema. Conclusion Our programme of research has repeatedly provided support for alterations of anterior brain functions as a significant factor in the influence of hypnosis in hypnotizable subjects, and one that is not seen in subjects of low hypnotizability who are in receipt of the same instructions. In order to avoid misunderstanding, it has been emphasized throughout that the results in no way imply a global inhibition, disconnection or deactivation of the frontal lobes. The ERP study which sampled both earlier and later stages of hypnosis indicated that alterations may change with the temporal course of hypnosis. Left frontal functions appear to be selectively more prone to alteration than right-sided functions, but given the cognitive flexibility/neural efficiency of the hypnotizable subject some frontal functions bilaterally may conceivably be enhanced depending on the instructions of hypnosis given. Consideration of the literature on hypnotizability (Heap et al., 2004) discloses that this is the first time that neural efficiency as defined by the fMRI BOLD index has been introduced as a construct to distinguish high from low hypnotizability. Neural efficiency can be seen to amplify the construct of cognitive flexibility. As has been developed elsewhere (Gruzelier, 2002a) a highly flexible nervous system may under some circumstances be a vulnerable one and reflect vulnerabilities for pathology through both imbalances in the internal milieu and susceptibilities to psychological stressors. The evidence of associations with the schizotypal personality provides one such example (Jamieson and Gruzelier, 2001; Gruzelier, De Pascalis, Jamieson, Laidlaw, Naito, Bennett and Dwivedi, 2004). Recent evidence shows strong relations in medical students between hypnotizability and a range of personality variables, including low self-directedness, schizotypy, affective distress, anxiety and depression, as well as with altered states of consciousness, imaginative involvement, and self-transcendence (Laidlaw, Dwivedi, Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 28 John Gruzelier Naito and Gruzelier, 2004). This is not the first time that an apparent predisposition to psychopathology has been theorized to construe evolutionary benefits such as provocative theories of the evolution of man and culture through schizotypal characteristics (Jaynes, 1976; Horrobin, 2001). Finally another dynamic reviewed here concerns the enhancement of some right hemispheric processes in hypnosis. Right hemispheric involvement in hypnosis has been a popular though controversial view amongst hypnotherapists and scientists (Crawford and Gruzelier, 1992), and has provided a highly appropriate metaphor in the clinic (Pedersen, 1984). Right hemisphericity as a trait of hypnosis was also popular, but for this we found no support (Gruzelier, 1998). In some groves of academe lateralization is anathema following journalistic popularization of theories of hemispheric specialization. Our results (Gruzelier et al., 1984; Jutai et al., 1993; McCormack and Gruzelier, 1993; Egner et al., 2005) should not be trivialized as evidence for simplistic binary left-right dynamics. The brain consists of functional networks extending bilaterally, and with excitatory as well as inhibitory dynamics, both of which require neuronal activation and metabolism. And while on this theme, be mindful too, that altered states of consciousness have only very recently been considered by some a respectable subject of scientific investigation in cognitive neuroscience (see Dietrich, 2003; Vaitl, Birbaumer, Gruzelier, Jamieson, Kotchoubey, Kübler, Lehmann, Miltner, Ott, Pütz, Sammer, Strauch, Strehl, Wackermann and Weiss, 2005, for some restoration to favour). Currently then our evidence of disconnectivities of the left lateral prefrontal cortex in our fMRI/EEG study contribute to growing evidence of a neurophysiological basis to hypnosis and other altered states of consciousness (see also Rainville et al., 1999; Halligan, Athwal and Oakley, 2000; Kosslyn, Thompson and Constantini-Ferrando, 2000; Spiegel, 2003). Much further research is required to elucidate the exact nature of the processes involved. Acknowledgement Written while in receipt of EU New Information Technologies grant: Presenccia; Application, Creative Presence States. References Banyai EI, Hilgard ER (1976) A comparison of active-alert hypnotic induction with traditional relaxation induction. Journal of Abnormal Psychology 85: 218–24. Bechara A, Damasio A, Damasio H, Anderson S (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15. Bechara A, Damasio H, Damasio AR (2000) Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex 10: 295–307. Bechara A, Damasio H, Damasio AR, Lee, GP (1999) Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience 19: 5473–81. Bechara A, Damasio H, Tranel D, Damasio AR (1997) Deciding advantageously before knowing the advantageous strategy. Science 275: 1293–5. Bechara A, Tranel D, Damasio H, Damasio A (1996) Failure to respond automatically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex 6: 215–25. Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences 8: 539–46. Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch Frontal connectivity and hypnosis 29 Botvinick M, Nystrom LE, Fissell L, Carter CS, Cohen JD (1999) Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402: 179–81. Bowers KS (1992) Imagination and dissociation in hypnotic responding. International Journal of Clinical and Experimental Hypnosis 40: 253–75. Buck R (1994) The neuropsychology of communication; spontaneous and symbolic aspects. Journal of Pragmatics 22: 265–78. Carter CS, MacDonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD (2000) Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences USA 97: 1944–8. Cavada C, Schultz W (2000) The mysterious orbitofrontal cortex. Foreword. Cerebral Cortex 10: 205. Cikurel K, Gruzelier J (1990) The effect of an active-alert hypnotic induction on lateral asymmetry in haptic processing. British Journal of Experimental and Clinical Hypnosis 7: 17–25. Crawford HJ (1989) Cognitive and physiological flexibility: multiple pathways to hypnotic responsiveness. In: V Ghorghui, P Netter, H Eysenck, R Rosenthal (eds) Suggestion and Suggestibility: Theory and Research. Berlin: Springer-Verlag, 155–68. Crawford HJ, Gruzelier JH (1992) A midstream view of the neuropsychophysiology of hypnosis: recent research and future directions. In: W Fromm, M Nash (eds) Hypnosis, Research Develrd opments and Pespectives, 3 edn. New York: Guildford Press, 227–66. Critchley HD, Elliott R, Mathias CJ, Dolan RJ (2000) Neural activity relating to generation and representation of galvanic skin conductance responses; a functional magnetic resonance imaging study. Journal of Neuroscience 20: 3033–40. Croft RJ, Williams JD, Haenschel C, Gruzelier JH (2002) Pain perception and 40 Hz oscillations: the effect of hypnotic analgesia. International Journal of Psychophysiology 41: 101–8. Damasio AR (1994) Descartes’ Error: Emotion, Rationality and the Human Brain. New York: Putnam. De Benedittis G, Sironi VA (1988) Arousal effects of deep brain stimulation in hypnosis, International Journal of Clinical and Experimental Hypnosis 36: 96–106. Derbyshire SW, Whalley MG, Stenger VA, Oakley DA (2004) Cerebral activation during hypnotically induced and imagined pain. Neuroimage 23: 392–401. Dietrich A (2003) Functional neuroanatomy of altered states of consciousness: the transient hypofrontality hypothesis. Consciousness and Cognition 12: 231–56. Dolan RJ (1999) On the neurophysiology of morals. Nature Neuroscience 2: 927–9. Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H (2000) A neural basis for general intelligence. Science 289: 457–60. Egner T, Jamieson G, Gruzelier JH (2005) Hypnosis decouples cognitive control from conflict monitoring processes of the frontal lobe. NeuroImage 27: 969–78. Evans FJ (1991) Hypnotisability: individual differences in dissociation and the flexible control of psychological processes. In: SJ Lynn, JW Rhue (eds) Theories of Hypnosis. London: Guildford Press, 144–68. Evans FJ, Graham C (1980) Subjective random number generation and attention deployment during acquisition and over learning of a motor skill. Bulletin of the Psychonomic Society 15: 391–4. Faymonville ME, Laureys S, Degueldre C, DelFiore G, Luxen A, Franck G, Lamy M, Maquet P (2000) Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology 92: 1257–67. Gray, J (1982) The Neuropsychology of Anxiety. Oxford: Oxford University Press. Gray JR, Thompson PM (2004) Neurobiology of intelligence: science and ethics. Nature Reviews neuroscience 5: 471–82. Gruzelier JH (1988) The neuropsychology of hypnosis. In: M Heap (ed.) Hypnosis: Current Clinical, Experimental and Forensic Practices. London: Croom Helm, 68–76. Gruzelier JH (1990) Neuropsychological investigations of hypnosis: cerebral laterality and beyond. In: R Van Dyck, PH Spinhoven, AJW Van der Does (eds) Hypnosis: Theory, Research and Clinical Practice. Amsterdam: Free University Press, 38–51. Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 30 John Gruzelier Gruzelier JH (1998) A working model of the neurophysiology of hypnosis: a review of the evidence. Contemporary Hypnosis 15:3–21. Gruzelier JH (2000a) Redefining hypnosis: theory, methods and integration. Contemporary Hypnosis 17: 51–70. Gruzelier JH (2000b) Unwanted effects of hypnosis: a review of the evidence and its implications. Contemporary Hypnosis 17: 163–93. Gruzelier JHth (2002a) New insights into the nature of hypnotisability. In: Beyond and Behind the Brain, 4 Bial Symposium, Fundacao Bial, 275–92. Gruzelier JH (2002b) The role of psychological intervention in modulating aspects of immune function in relation to health and well being. International Review of Neurobiology 52: 383–417. Gruzelier JH (2004) Neurophysiologische erorterung der ungunstigen effekte der hypnose unter besonderer berucksichtigung der buhnen-hypnose. Hypnose und Kognition (HyKog) 21: 225–59. Gruzelier JH, Venables PH (1972): Skin conductance orienting activity in a heterogeneous sample of schizophrenics: possible evidence of limbic dysfunction. Journal of Nervous and Mental Disease 155: 277–87. Gruzelier JH, Allison J, Conway A (1988) A psychophysiological differentiation between hypnosis and the simulation of hypnosis. International Journal of Psychophysiology 6: 331–8. Gruzelier JH, Brow TD (1985) Psychophysiological evidence for a state theory of hypnosis and susceptibility. Journal of Psychosomatic Research 29: 287–302. Gruzelier JH, Brow TD, Perry A, Rhonder J, Thomas M (1984) Hypnotic susceptibility: a lateral predisposition and altered cerebral asymmetry under hypnosis. International Journal of Psychophysiology 2: 131–9. Gruzelier J, De Pascalis V, Jamieson G, Laidlaw T, Naito A, Bennett B, Dwivedi P (2004) Relations between hypnotisability and psychopathology revisited. Contemporary Hypnosis 21: 169–70. Gruzelier JH, Gray M, Horn P (2002) The involvement of frontally modulated attention in hypnosis and hypnotic susceptibility: cortical evoked potential evidence. Contemporary Hypnosis 19: 179–89. Gruzelier JH, Phelan M (1991) Laterality-reversal in a lexical divided visual field task under stress. International Journal of Psychophysiology 11: 267–76. Gruzelier JH, Warren K (1993) Neuropsychological evidence of left frontal inhibition with hypnosis. Psychological Medicine 23: 93–101. Haier RJ, Siegel BV, Maclachlan A, Soderling E, Lottenberg S, Buchsbaum MS (1992) Regional glucose metabolic changes after learning a complex visuospatial/motor task: a positron emission tomographic study. Brain Research 570: 134–43. Haier RJ, Siegel BV, Tang C, Abel, L, Buchsbaum MS (1992) Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence 16: 415–26. Halligan PW, Athwal BS, Oakley DA (2000) Imaging hypnotic paralysis: implications for conversion hysteria. The Lancet 355(9208): 986–7. Heap M, Brown RJ, Oakley DA (2004) The Highly Hypnotizable Person. London: Routledge. Hilgard ER (1965) Hypnotic Susceptibility. New York: Harcourt Brace & World. Hilgard JR, Hilgard ER, Newman M (1961) Sequaelae to hypnotic induction with special reference to earlier chemical anesthesia. The Journal of Nervous and Mental Disease 133: 461–78. Horrobin D (2001) The Madness of Adam and Eve. London: Bantam Press. Hugdahl K (1995) Classical conditioning and implicit learning: the right hemisphere hypothesis. In: RJ Davidson, K Hugdahl (eds) Brain Asymmetry. Cambridge, MA: MIT Press. Jamieson G, Gruzelier JH (2001) Hypnotic susceptibility is positively related to a subset of schizotypy items. Contemporary Hypnosis 18: 32–7. Jamieson GA, Sheehan PW (2004) An empirical test of Woody and Bower’s dissociated control theory of hypnosis. International Journal of Clinical and Experimental Hypnosis 52: 232–49. Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch Frontal connectivity and hypnosis 31 Jaynes J (1976) The Origin of Consciousness in the Breakdown of the Bicameral Mind. London: Penguin Books. Jutai J, Gruzelier JH, Golds J, Thomas M (1993) Bilateral auditory-evoked potentials in conditions of hypnosis and focused attention. International Journal of Psychophysiology 15: 167–76. Kaiser J, Barker R, Haenschel C, Baldeweg T, Gruzelier JH (1997) Hypnosis and event-related potential correlates of error processing in a stroop-type paradigm: a test of the frontal hypothesis. International Journal of Psychophysiology 27: 215–22. Kallio S, Revonsuo A, Hamalainen H, Markela J, Gruzelier J (2001) Changes in anterior attentional functions and word fluency associated with hypnosis. International Journal of Clinical and Experimental Hypnosis 49: 95–108. Keenan JP, Wheeler MA, Gallup JG, Pasual-Leone A (2000) Self-recognition and the right prefrontal cortex. Trends in Cognitive Sciences 4: 338–44. Kleinhauz M, Behan B (1981) Misuses of hypnosis: a medical emergency and its treatment. The International Journal of Clinical and Experimental Hypnosis 39: 148–61. Kleinhauz M, Dreyfuss DA, Behan B, Goldberg T, Azikri D (1979) Some after-effects of stage hypnosis: a case study of psychopathological manifestations. The International Journal of Clinical and Experimental Hypnosis 27: 219–26. Koechlin E, Ody C, Kouneiher F (2003) The architecture of cognitive control in the human prefrontal cortex. Science 302: 1181–5. Kosslyn SM, Thompson WL, Constantini-Ferrando MF (2000) Hypnotic visual illusion alters brain color processing. American Journal of Psychiatry 45: 327–33. Laidlaw TM, Dwivedi P, Naito A, Gruzelier JH (2004) Low self-directedness (TCI), mood, schizotypy and hypnotic susceptibility. Personality and Individual Differences 39: 469–80. MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–8. McCormack K, Gruzelier JH. (1993) Cerebral asymmetry and hypnosis: a signal detection analysis of divided visual field stimulation. Journal of Abnormal Psychology 102: 352–7. Neubauer AC, Grabner RH, Freudenthaler HH, Beckman JF, Guthke J (2004) Intelligence and individual differences in becoming neurally efficient. Acta Psychologica 116: 55–74. Nordby H, Hugdahl K, Jasiukaitis P, Spiegel D (1999) Effects of hypnotizability on performance of a Stroop task and event-related potentials. Perceptual and Moter Skills 88: 819–30. Page RA, Handley GW (1993) The effect of preventive measures in reducing after effects to hypnosis. American Journal of Clinical Hypnosis 36(1): 26–37. Passingham RE, Toni I, Rushworth MFS (2000) Specialisation within the prefrontal cortex: the ventral prefrontal cortex and associative learning. Experimental Brain Research 133: 103–13. Pedersen D (1984) Hypnosis and the right hemisphere. Proceedings of the British Society of Medical and Dental Hypnosis 5: 2–14. Pizzagali, D, Regard M, Lehmann D (1999) Rapid emotional processing in the human right and left brain hemispheres. NeuroReport 10: 2690–1. Pribram K, McGuiness D (1975) Arousal, activation and effort in the control of attention. Psychological Review 82: 116–30. Raab J, Gruzelier J (1994) A controlled investigation of right hemispheric processing enhancement after restricted environmental stimulation (REST) with floatation. Psychological Medicine 24: 457–62. Rainville P, Duncan GH, Price DD, Carrie B, Bushnell MC (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277: 968–71. Rainville P, Hofbauer RK, Bushnell MC, Duncan GH, Price DD (2000) Hypnosis modulates activity in brain structures involved in the regulation of consciousness. Journal of Cognitive Neuroscience 14: 887–901. Rainville P, Hofbauer R K, Paus T (1999) Cerebral mechanisms of hypnotic induction and suggestion. Journal of Cognitive Neuroscience 11: 110–25. Santarcangelo EL, Sebastiani, L (2004) Hypnotisability as an adaptive trait. Contemporary Hypnosis 21: 3–13. Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch 32 John Gruzelier Schoenbaum G, Chiba AA, Gallagher M (1998) Oribitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience 1(2): 155–9. Schore A (1994) Affect Regulation and the Origin of the Self. The Neurobiology of Affective Development. Hillsdale, NJ: Erlbaum. Schore A (1997) Early organisation of the nonlinear right brain and development of a predisposition to psychiatric disorders. Development and Psychopathology 9: 595–631. Shallice T (2002) Fractionation of the supervisory system. In: DT Stuss, RT Knight (eds) Principles of Frontal Lobe Function. Oxford: Oxford University Press, 261–77. Shames V, Bowers PG (1992) Hypnosis and creativity. In: E Fromm and M Nash (eds) Contemporary Hypnosis Research. New York: Guildford Press, 334–63. Spiegel D (2003) Negative and positive visual hypnotic hallucinations: attending inside and outside. International Journal of Clinical and Experimental Hypnosis 51: 130–46. Tallon-Baudry C, Bertrand O (1999) Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences 3: 151–62. Vaitl D, Birbaumer N, Gruzelier J, Jamieson G, Kotchoubey B, Kübler A, Lehmann D, Miltner WHR, Ott U, Pütz P, Sammer G, Strauch I, Strehl U, Wackermann J, Weiss T (2005) Psychobiology of altered states of Consciousness. Psychological Bulletin 13: 98–127. Woody EZ, Bowers KS (1994) A frontal assault on dissociated control. In: SJ Lynn, JW Rhue (eds) Dissociation: Clinical and Theoretical Perspectives. New York: Guilford Press, 52–79. Woody E, Sadler P (1998) On reintegrating dissociated theories: comment on Kirsch and Lynn (1998) Psychological Bulletin 123: 192–7. Zeman A (2002) Consciousness: A User’s Guide. London: Yale University Press. Address for correspondence: John Gruzelier Department of Psychology Goldsmiths College New Cross London SE14 6NW UK Email: [email protected] Copyright © 2006 British Society of Experimental & Clinical Hypnosis Published by John Wiley & Sons, Ltd Contemp. Hypnosis 23: 15–32 (2006) DOI: 10.1002/ch