* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture Topic: Fatty Acid Synthesis
Photosynthetic reaction centre wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Catalytic triad wikipedia , lookup
Point mutation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Proteolysis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nitrogen dioxide poisoning wikipedia , lookup
Plant nutrition wikipedia , lookup
Metalloprotein wikipedia , lookup
Genetic code wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Biochemistry wikipedia , lookup
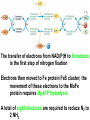
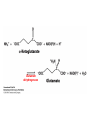
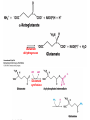
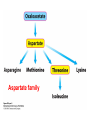
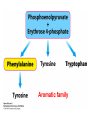
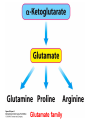
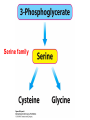
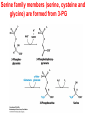
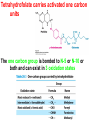
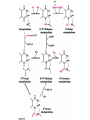
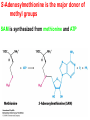
10-21-11 Nitrogen Metabolism 1. Nitrogen Fixation 2. Amino Acid Biosynthesis Nitrogen is an essential element found in proteins, nucleic acids and many other molecules Biologically available nitrogen is scarce Nitrogen incorporation begins with fixation (reduction) of N2 by prokaryotic microorganisms to form ammonia (NH3) Nitrogen supply is often the rate-limiting factor in plant growth Nitrogen is assimilated by conversion into the amide group of glutamine, which can then be used for other carbon-containing compounds (e.g., amino acids) The nitrogen cycle is the complex process by which nitrogen is transferred throughout the living world Amino acid metabolism is an important process involving nitrogen Animals can only produce half of the amino acids required (nonessential amino acids); others must be obtained from diet (essential amino acids) Transamination reactions dominate amino acid metabolism (aminotransferases or transaminases) Nitrogen fixation occurs industrially via the Haber reaction, accounting for 25% of earth’s yearly fixed nitrogen production as fertilizer N2 + 3 H2 2 NH3 500oC 300 atmospheres Lightning strikes and ultraviolet light produce another 15% of earth’s fixed nitrogen Biological nitrogen fixation, the cellular method to execute this thermodynamically favorable reaction, produces 60% of earth’s fixed nitrogen Nitrogen fixation is only possible by a limited number of species Among the most prominent nitrogen-fixing species are free-living bacteria, cyanobacteria and symbiotic bacteria Organisms such as Azotobacter vinelandii, Anabaena azollae and Rhizobium species Energy requirement is extremely high: 16 ATP to form two NH3 from one N2 The Nitrogen Fixation Reaction All species that can fix nitrogen contain the nitrogenase complex Consists of two proteins dinitrogenase reductase and dinitrogenase Dinitrogenase reductase (Fe Prot.) passes electrons from NAD(P)H one at a time to dinitrogenase Uses 4Fe-4S cluster and MgATP-binding site Dinitrogenase (MoFe protein) catalyzes the reaction N2 + 8H+ + 8e- 2NH3 + H2 Uses P cluster [8Fe-7S] and MoFe cofactor prosthetic groups dinitrogenase dinitrogenase reductase The transfer of electrons from NAD(P)H to ferredoxin is the first step of nitrogen fixation Electrons then moved to Fe protein FeS cluster; the movement of these electrons to the MoFe protein requires MgATP hydrolysis A total of eight electrons are required to reduce N2 to 2 NH3 Nitrogen Assimilation Nitrogen assimilation is the incorporation of inorganic nitrogen compounds into organic molecules Nitrogen assimilation begins in the roots of plants NH4+ (from soil or root nodules) or NO3(nitrate) is incorporated into amino acids If nitrate is the nitrogen source, a two-step reaction is used to first convert it to NH4+ Glutamate dehydrogenase synthesizes glutamate from NH4+ and a-ketoglutarate Glutamate dehydrogenase Glutamine synthetase catalyzes the ATPdependent reaction of glutamate with NH4+ to form glutamine Living organisms differ in their ability to synthesize amino acids Many plants and microbes can synthesize all of the amino acids, while mammals cannot Reactions of Amino Groups Once amino acids have entered the cell, their amino groups are available for synthetic reactions Usually via transamination or direct incorporation Transamination - Aminotransferases are responsible for the reactions are found in cytoplasm and mitochondria oxaloacetate and pyruvate are converted to amino acids by transamination oxaloacetate + glutamate aspartate + a-ketoglutarate pyruvate + glutamate alanine + a-ketoglutarate Most aminotransferases use glutamate as the amino group donor The glutamate/a-ketoglutarate pair play an important role in nitrogen metabolism Transamination reactions require the coenzyme pyridoxal-5ʹ-phosphate (PLP), which is derived from pyridoxine (vitamin B6) PLP accepts an amino group to form cofactor PMP Direct incorporation of ammonium ions into organic molecules: Two methods 1) Reductive amination of a-keto acids 2) Formation of the amides of aspartic and glutamic acid Glutamate dehydrogenase catalyzes the direct amination of a-ketoglutarate Ammonium ions are also incorporated into cell metabolites by the formation of glutamine, the amide of glutamate (glutamine synthetase) Glutamate dehydrogenase Glutamate synthetase Synthesis of the Amino Acids Amino acids differ from other biomolecules in that each member is synthesized in a unique pathway On the basis of the similarities in their synthetic pathways, they can be grouped into six families Glutamate, serine, aspartate, pyruvate, the aromatics and histidine The amino acids in each family are ultimately derived from one precursor molecule Amino acids are made from intermediates of major pathways Amino acids can be grouped on the basis of their metabolic origins, as follows Pathway origins are indicated in blue Amino acid precursors of other amino acids in yellow Essential amino acids in humans in bold Aspartate family Aromatic family Pyruvate family Histidine family Glutamate family Serine family Serine family members (serine, cysteine and glycine) are formed from 3-PG Tetrahydrofolate carries activated one carbon units The one carbon group is bonded to N-5 or N-10 or both and can exist in 3 oxidation states Tetrahydrofolate is critical for DNA replication and cell growth Anti-cancer drugs are often compounds that inhibit the ability to regenerate tetrahydrofolate and thus slow cancer cell growth Tetrahydrofolate is important in development of the fetal nervous system; deficiency can cause spina bifida and anencephaly Tetrahydrofolate is derived from folic acid (Vit. B9) S-Adenosylmethionine is the major donor of methyl groups SAM is synthesized from methionine and ATP The activated methyl group on SAM makes it a strong methyl group donor After methyl group transfer S-adenosyl homocysteine is hydrolyzed to adenosine and homocysteine Methionine is regenerated by transfer of a methyl group to homocysteine from N5-methyltetrahydrofolate methionine synthase The activated methyl cycle High homocysteine levels correlate with vascular disease The most common genetic basis for high homocysteine levels is mutation of the gene for cystathionine b-synthetase High homocysteine levels may: Damage cells lining blood vessels Increase growth of vascular smooth muscle Increase oxidative stress Vitamin treatments are sometimes effective in reducing homocyteine levels Pyridoxal phosphate is needed by cystathionine b-synthetase THF and vitamin B12 support the methylation of homocysteine to methionine Over expression of cystathionine beta-synthetase leads to decreased homocysteine levels in the blood and may contribute to Down symdrome