* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Amyloplasts and Vacuolar Membrane Dynamics in
Survey
Document related concepts
Cell nucleus wikipedia , lookup
Signal transduction wikipedia , lookup
Extracellular matrix wikipedia , lookup
Programmed cell death wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell membrane wikipedia , lookup
Endomembrane system wikipedia , lookup
Cytokinesis wikipedia , lookup
Transcript
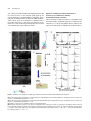
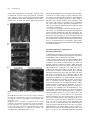
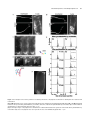
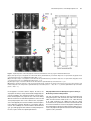
The Plant Cell, Vol. 17, 548–558, February 2005, www.plantcell.org ª 2005 American Society of Plant Biologists Amyloplasts and Vacuolar Membrane Dynamics in the Living Graviperceptive Cell of the Arabidopsis Inflorescence Stem W Chieko Saito, Miyo T. Morita, Takehide Kato, and Masao Tasaka1 Graduate School of Biological Sciences, Nara Institute of Science and Technology, Ikoma, Nara 630-0101, Japan We developed an adequate method for the in vivo analysis of organelle dynamics in the gravity-perceptive cell (endodermis) of the Arabidopsis thaliana inflorescence stem, revealing behavior of amyloplasts and vacuolar membranes in those cells. Amyloplasts in the endodermis showed saltatory movements even before gravistimulation by reorientation, and these movements were confirmed as microfilament dependent. From our quantitative analysis in the wild type, the gravityoriented movement of amyloplasts mainly occurred during 0 to 3 min after gravistimulation by reorientation, supporting findings from our previous physiological study. Even after microfilament disruption, the gravity-oriented movement of amyloplasts remained. By contrast, in zig/sgr4 mutants, where a SNARE molecule functioning in vacuole biogenesis has been disrupted, the movement of amyloplasts in the endodermis is severely restricted both before and after gravistimulation by reorientation. Here, we describe vacuolar membrane behavior in these cells in the wild-type, actin filament–disrupted, and zig/sgr4 mutants and discuss its putatively important features for the perception of gravity. We also discuss the data on the two kinds of movements of amyloplasts that may play an important role in gravitropism: (1) the leading edge amyloplasts and (2) the en mass movement of amyloplasts. INTRODUCTION Higher plants cannot escape from the place where they germinate and settle, even if the environmental conditions drastically change. Plants have developed many mechanisms during the course of evolution to survive in such circumstances by changing growth direction or architecture of their body shape. Typical of such growth responses, gravitropism is one of most prominent phenomena and has been the focus of studies on many plant species over a long period of time (Knight, 1806; Sack, 1991; Fukaki et al., 1996b; Chen et al., 1999). In higher plants, shoots and roots generally show negative and positive gravitropism, respectively. This response ensures an appropriate positioning of tissues for efficient photosynthesis, gas exchange, and the supply of water and minerals. The gravitropic response in plants can be divided into four sequential steps: gravity perception, signal formation in the gravity perceptive cell, intracellular and intercellular signal transduction, and an asymmetric cell elongation between the upper and lower sides of the responding organs (Tasaka et al., 1999). Some classical hypotheses have been supported by recent molecular genetical studies. For example, the starch-statolith 1 To whom correspondence should be addressed. E-mail m-tasaka@ bs.naist.jp; fax 81-743-72-5489. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Masao Tasaka ([email protected]). W Online version contains Web-only data. Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.026138. hypothesis is involved in the first step and proposes that the sedimenting amyloplasts, which contain dense starch granules, act in sensing the direction of gravity (Sack, 1991; Weise and Kiss, 1999). However, the mechanism functioning in gravity perception has not yet been characterized at the molecular level. A recent molecular approach using Arabidopsis thaliana revealed the graviperceptive cell in an inflorescence stem. We have isolated several shoot gravitropism (sgr) mutants, which are defective in the gravitropic response of inflorescence stems. In the Arabidopsis shoot, the epidermis, cortex, endodermis, and stele containing vascular tissues are arranged in a radially symmetrical manner. In the course of the study of sgr1 and sgr7, which are allelic to scarecrow and short-root, respectively, the endodermis has been revealed as essential for the gravitropism of the inflorescence stem (Fukaki et al., 1998; Tasaka et al., 1999). In addition, it is known that amyloplasts differentiate and sediment to the direction of gravity in the endodermis. These findings suggest that the endodermis in the inflorescence stem is essential for the first step of shoot gravitropism, namely the perception of gravity. The starch-statolith hypothesis was also supported by these results. Interestingly, some other sgr mutants, including sgr2, sgr3, and zig/sgr4, have been shown to still have an endodermis, although the sedimentation of amyloplasts in those cells is abnormal (Morita et al., 2002; Yano et al., 2003). These mutants show a gravitropism defect in the shoot exclusively (inflorescence stem and hypocotyls) and are normal in root gravitropism or phototropism. Surprisingly, responsible genes of these mutants are closely related to the biogenesis or the integrity of the vacuoles (Kato et al., 2002; Yano et al., 2003). Moreover, it is revealed that amyloplasts in the wild-type Subcellular Dynamics of the Graviperceptive Cell endodermis are almost completely enwrapped by a thin cytoplasmic layer and vacuolar membrane, whereas amyloplasts are left in the peripheral region of the cytoplasm of the endodermal cells in the mutants (Morita et al., 2002). When expressed under the control of the SCARECROW promoter (ProSCR), which drives expression in the endodermis, the corresponding wild-type genes restored gravitropism and amyloplast sedimentation in the mutant (Morita et al., 2002; Yano et al., 2003). These findings suggest that the early step of the gravitropism occurring in the endodermis is impaired in the mutants. They still retain, however, the ability for the latter steps, signal formation in the gravity perceptive cell, intracellular and intercellular signal transduction, and an asymmetric cell elongation between the upper and lower sides of the responding organs for tissue bending. These findings also suggest that vacuoles in the endodermis may be involved in the early step of gravitropism to some extent. In the gravity-perceptive cell of the root (columella cell), several detailed analyses of the subcellular dynamics have been performed after gravistimulation by reorientation (Legue et al., 1997; Fasano et al., 2001; Yoder et al., 2001), and it has been shown that amyloplasts actually sediment to the orientation of gravity (Yoder et al., 2001). In the shoot, however, only a few attempts have been made at visualizing the gravity-perceptive cell at a subcellular level (Johannes et al., 2001), and to our knowledge, no studies have tried to analyze the movement dynamics of the vacuolar membrane in the endodermal cell. Here, we describe amyloplast movement in the living endodermal cell of Arabidopsis inflorescence stems using a vertical stage microscope. Interestingly, they do not sediment statically but show saltatory movement. We also visualize the movement dynamics of the vacuolar membrane in the endodermal cells. Confocal laser scanning microscopy revealed where amyloplasts pass through. Treatment with a microfilament-disrupting drug severely affected the movement of amyloplasts before gravistimulation by reorientation, although the gravity-perceptive ability of the inflorescence stem segment still remained. In zig-1/sgr4-1 mutants, the movement of amyloplasts was severely affected, and their gravitropism was almost completely defective as shown previously. We will discuss the possible role of the gravityperceptive system in the dynamics of amyloplasts and the close interaction with the vacuolar membrane. This study opens the possibility of a new concept for gravity perception in the inflorescence stem. RESULTS Methods for the Observation of Organelle Dynamics and Subcellular Events in a Living Endodermal Cell A transgenic plant containing green fluorescent protein (GFP)marked amyloplasts in its endodermis (pspt3-6) was generated by transforming into wild type an expression construct carrying a GFP sequence fused to a plastid-targeted transit peptide, under the control of ProSCR (Figures 1A and 1B). Amyloplasts were still detectable using autofluorescence even in the nontransgenic plant (wild type); however, a clearer image of amylo- 549 Figure 1. Visualization of Organelle Dynamics in the Endodermis. (A) Construction for the endodermal-specific expression of plastid-GFP (top, for pspt3-6 line) and tonoplast-GFP (bottom, for psgt4-1 line) markers. (B) Endodermal-specific expression in the inflorescence stem observed in the longitudinal section. Left, blue light–excited fluorescence image; right, Bright-field image. En, endodermal cell files. Bar ¼ 20 mm. (C) Epifluorescence image of endodermal cells fixed, embedded, sectioned (1 mm thick), and stained with DiOC6. Bar ¼ 5 mm. (D) Transmission electron micrograph of an ultrathin section of endodermal cells. Bar ¼ 2 mm. (E) Epifluorescence image of living endodermal cells of the pspt3-6 lines observed under blue light excitation with a wide-range emission filter set. Amyloplasts were visualized by the expression of plastid-targeted GFP. Chloroplasts in cortex cells or stele were also visualized by their autofluorescence. Co, cortex cell file; En, endodermal cell file; St, stele. Bar ¼ 5 mm. (F) Epifluorescence image of living endodermal cells of the psgt4-1 line under blue light excitation with a band-path filter set. Vacuolar membrane in the endodermal cells was visualized by GFP-gTIP expression under ProSCR. Bar ¼ 5 mm. plasts was obtained in the transgenic line (Figure 1E). The images are comparable to those made from a fixed sample section (Figures 1C and 1D). ProSCR and GFP were further applied for the visualization of the vacuolar membrane. GFP and g-TIP (tonoplast intrinsic protein g), a marker of the lytic vacuole (Maurel 550 The Plant Cell et al., 1993), were fused and expressed in wild-type plants under the control of ProSCR. In the transgenic plant (psgt4-1), the vacuolar membrane is visualized within the endodermis specifically with the band-path filter set (Figure 1F; NIBA; Olympus, Tokyo, Japan). As far as we examined, no significant leak of the marker molecules to other layers was detected in the inflorescence stem, and both pspt3-6 and psgt4-1 lines showed a normal gravitropic response (data not shown). Dynamics of Amyloplasts in Endodermal Cells of the Inflorescence Stem before and after Gravistimulation by Reorientation First, we wanted to examine the dynamics of amyloplasts using time-lapse imaging. Sequential images were acquired at 10-s intervals for 5 to 20 min. Amyloplasts did not sediment statically but moved within the endodermal cell dynamically, as Figure 2. Dynamics of the Amyloplasts in a Wild-Type Endodermal Cell before and after Gravistimulation by Reorientation. (A) Left, time-lapse images of amyloplasts at 1-min intervals before gravistimulation by reorientation. Right, trace of amyloplasts. Each color represents the trace of an individual amyloplast. Bars ¼ 5 mm. (B) Top, time-lapse images of amyloplasts at 1-min intervals after gravistimulation by reorientation. Bottom, trace of amyloplasts. Each color represents the trace of an individual amyloplast. Bars ¼ 5 mm. (C) Definition of the axis for the quantitative analysis of amyloplast dynamics. (D) Histograms showing the frequency of the speed of amyloplasts tracks, extracted to Dx and Dy components in the wild type. Braces represent increased gravity-oriented movements of the amyloplasts. Asterisks show the populations of the positive Dy components with large absolute values (>3 mm/10 s). Total number subjected to the analysis before gravistimulation by reorientation (tracks n ¼ 825, cells n ¼ 3) and after gravistimulation by reorientation (tracks n ¼ 443, n ¼ 3). Subcellular Dynamics of the Graviperceptive Cell shown by a time-lapse movie (see Supplemental Movie 2 online, x100 speed) and the tracing of movement (Figure 2A, right). They moved not only along the peripheral area, but also through the central region of the endodermal cell very frequently. These dynamic movements were observed continuously even after 20 min (up to 40 min maximum) after preparation of samples. After gravistimulation by reorientation, the amyloplasts changed their localization pattern drastically and some of them reached the lower end of the cell (Figure 2B, 3 min). They did not stay at the basal end but continued to move around (see Supplemental Movie 3 online). All of the movements of amyloplasts were not directly toward the gravity orientation during this short time period. Some amyloplasts appeared to actually move along a newly applied gravity orientation and others not, as shown by the trace of each amyloplast, after gravistimulation by reorientation (Figure 2B, bottom). Gravity-Oriented Movement of Amyloplasts Occurs during the First Three Minutes after Reorientation Attempts were made to analyze the movements of amyloplasts quantitatively and statistically. Tracks of some amyloplasts were traced continuously at 10-s intervals using time-lapse images (Figures 2A, right, and 2B, bottom). To extract gravityoriented movement, each movement at every 10-s interval was dissected into two components, one horizontally (Dx) and the other vertically (Dy), as shown in Figure 2C. Taking time dependency of the movement into account, the time period was divided into 0 to 1 min, 1 to 2 min, 2 to 3 min, 3 to 4 min, and 4 to 5 min after application of gravistimuli. Figure 2D shows histograms of the frequency of Dx and Dy components. Before gravistimulation by reorientation, histograms of both Dx and Dy showed a Gaussian distribution–like shape and were symmetric to the axis of Dx ¼ 0 and Dy ¼ 0, respectively. After gravistimulation by reorientation, in the time period of 0 to 1 min, 1 to 2 min, and 2 to 3 min, clear asymmetric distributions 551 of the Dy components were detected (Figure 2D, braces). The mean values of Dy during this period are statistically significantly increased compared with the mean value of Dy before gravistimulation by reorientation (Welch’s t test, one-tailed, P < 0.05). During the time period of 1 to 2 min and 2 to 3 min, small but certain populations of the positive Dy components with large absolute values were also detected (asterisks, >3 mm/ 10 s). This population was 0.9, 2.3, 2.6, 0, and 1.37% in 0 to 1 min, 1 to 2 min, 2 to 3 min, 3 to 4 min, and 4 to 5 min, respectively (before reorientation, 1.5%). The average value of Dy components from the same data set of the tracks is shown in Table 1. During 0 to 3 min, the averaged speed to the gravity is 1.05 mm/min to 1.42 mm/min. These quantitative results indicate that the gravity-oriented movements of amyloplasts (both the mass translocation to gravity and the positive Dy component with large absolute value) actually occurred after gravistimulation by reorientation. The mean values of Dx in the time periods of 2 to 3 min and 3 to 4 min are also statistically significantly increased compared with the mean value of Dx before gravistimulation by reorientation (Welch’s t test, onetailed, P < 0.05). Amyloplasts Are in the Transvacuolar Strand, Both before and after Gravistimulation by Reorientation It has been revealed using electron microscopy that the amyloplasts in the endodermal cells are surrounded almost completely by a thin layer of cytoplasm and the vacuolar membrane (Morita et al., 2002). The narrow areas of cytoplasm enclosed by the vacuolar membrane and continuous to the peripheral cytoplasm have been referred to as ‘‘transvacuolar strands.’’ It was expected that amyloplasts move through these transvacuolar strands before or after gravistimulation by reorientation. Vacuolar membranes in the endodermal cell are visualized well by expressing the GFP-g-TIP fusion molecule driven under ProSCR. As shown in Figure 1F, the vacuolar membrane in the endodermal cell emitted fluorescence in the psgt4-1 line, but the strand-like structure was not observed so Table 1. Averaged Speed of Amyloplasts (mm/min) Extracted to Dx and Dy Components þLatB Wild Type Before After applying gravistimuli 0 1 2 3 4 to to to to to 1 2 3 4 5 min min min min min zig-1/sgr4-1 Dx Dy n Dx Dy n Dx Dy n 0.1 6 5.9 0.2 6 6.7 825 0.12 6 2.1 0 6 2.2 404 0.1 6 3.4 0.4 6 4.3 869 0.03 0.1 1.05* 1.21* 1.2 1.05* 1.42* 1.11* 0.4 0.1 117 87 76 90 73 0.72* 0.40 0.44 0.41* 0.31 1.72* 0.94* 0.76* 0.86* 0.11 125 115 97 93 90 0.3 0.1 0.14 0.03 0.25 0.46 0.19 0.4 0.49 0.25 102 102 99 96 91 6 6 6 6 6 5.5 4.6 3.6 5.6 4.8 6 6 6 6 6 5.4 6.1 5.4 4.5 5.3 6 6 6 6 6 2.6 3.4 2.9 3.1 1.9 6 6 6 6 6 3.2 5.0 3.7 3.1 2.4 6 6 6 6 6 2.5 3.1 3.0 3.8 2.0 6 6 6 6 6 2.2 2.0 2.8 3.2 1.6 The averaged speed was calculated from the same data set that was used in the histograms shown in Figures 2D, 4E, and 5D (cell number, n ¼ 3, total tracks used in the calculation was represented in column n). The axis was defined as shown in Figure 2C. For the direction of movements along Dx and Dy, positive values mean right or down, and negative values indicate left or up, respectively. 6, standard deviations. Asterisks show the time period of the mean values of Dx or Dy statistically significantly increased compared with the mean value of Dx or Dy before gravistimulation by reorientation (Welch’s t test, one-tailed, P < 0.05). LatB, latrunculin B. 552 The Plant Cell clearly using epifluorescence microscopy. However, using a high-speed confocal scanner unit (CSU-10; Yokogawa, Tokyo, Japan), a complex network of the transvacuolar strands and some dilated areas, where amyloplasts are expected to localize, were observed both before (Figure 3A) and after (Figure 3B) gravistimulation by reorientation. From time-lapse confocal laser scanning images, vacuolar membranes were also shown to be quite dynamic even before gravistimulation by reorientation (see Supplemental Movies 4 and 5 online). A silhouette of the vacuolar membrane often showed the same size, shape, and number of amyloplasts in the endodermal cell. These images strongly suggest that amyloplasts move within the transvacuolar strands both before and after gravistimulation by reorientation. To confirm that amyloplasts translocate through transvacuolar strands, we visualized both amyloplasts autofluorescing and the vacuolar membrane (by GFP-gTIP expression) in the same cell of the psgt4-1 line with a very short time difference by changing the filter set. Figure 3C shows time-lapse images of the vacuolar membrane and amyloplasts observed alternately at 10-s intervals. Most of the GFP-gTIP signals were detected very close to the autofluorescence of amyloplasts. These images clearly indicate that amyloplasts move within the cell wrapped by the vacuolar membrane. The Saltatory Movement of Amyloplasts Is Microfilament Dependent Figure 3. Vacuolar Membrane Dynamics in a Wild-Type Endodermal Cell. (A) and (B) Time-lapse images of the vacuolar membrane visualized in the psgt4-1 line and captured by confocal laser scanning microscope before ([A], 10-s intervals) and after ([B], 5-s intervals) applying gravistimuli. Bars ¼ 5 mm. (C) Epifluorescence micrographs of amyloplasts and the vacuolar membrane in an endodermal cell captured very shortly after gravistimulation by reorientation. Left, amyloplasts observed by their autofluorescence with a wide-range filter set (WIB; Olympus); right, vacuolar membrane visualized by GFP-gTIP expression and observed under a band-path filter set (NIBA; Olympus). Bar ¼ 5 mm. To address the molecular basis of the movement of amyloplasts and to examine their association with gravitropism, the effect of the disruption of actin cytoskeleton was analyzed. After a 10-min treatment with the antimicrofilament drugs cytochalasin B and latrunculin B, the saltatory movements of amyloplasts were severely attenuated (data not shown). As reported previously (Yamamoto and Kiss, 2002), latrunculin B–treated inflorescence stems are able to respond to gravity. These findings were confirmed in this study after 14 h of treatment with latrunculin B (Figures 4A and 4B). It was also shown that the network of actin filaments was completely disrupted, as detected by Pro35S GFP talin (Figure 4B, right). The motility of amyloplasts was considerably reduced, and almost all of them were located at the basal region of the endodermal cell (Figure 4C; see Supplemental Movie 6 online). After gravistimulation by reorientation, however, some populations of amyloplasts actually sedimented, whereas others remained static (Figure 4D; see Supplemental Movie 7 online). Statistical analysis showed that the distribution of Dy components became steeper, indicating that the movements of amyloplasts were inhibited before gravistimulation by reorientation (Figures 2B and 4E). Positive Dy components showing large absolute values were detected (asterisks, >3 mm/10 s), and this population was 0.8, 2.5, 1.0, 0, and 0% in 0 to 1 min, 1 to 2 min, 2 to 3 min, 3 to 4 min, and 4 to 5 min, respectively (before reorientation, 0%). The average values of Dy components were comparable to those of the wild type (Table 1; 1.72 mm/min, 0.94 mm/min, 0.76 mm/min, and 0.86 mm/min in 0 to 1 min, 1 to 2 min, 2 to 3 min, and 3 to 4 min, respectively). The mean values of Dy during this period are statistically significantly increased compared with the mean value of Dy before gravistimulation by reorientation (Welch’s t test, one-tailed, P < 0.05). The mean values of Dx in the time periods of 0 to 1 min and 3 to 4 min are also statistically significantly increased Subcellular Dynamics of the Graviperceptive Cell 553 Figure 4. The Disruption of the Actin Cytoskeleton and Saltatory Movement of Amyloplasts and Retention of Gravitropism after Treatment with Latrunculin B. (A) and (B) Gravitropism assay of stem segment and F-actin filament in an endodermal cell visualized by GFP-tallin without (A) or with (B) latrunculin B treatment. Left top, 0 min after gravistimulation by reorientation; left bottom, 150 min after gravistimulation by reorientation; right, actin microfilament visualized by Pro35S-driven GFP-talin expression. Bars ¼ 5 mm. (C) Left, time-lapse images of amyloplasts in the endodermal cell of latrunculin B–treated stem segment at 1-min intervals before gravistimulation by reorientation. Right, trace of amyloplasts. Each color represents the trace of an individual amyloplast. Bars ¼ 5 mm. 554 The Plant Cell compared with the mean value of Dx before gravistimulation by reorientation (Welch’s t test, one-tailed, P < 0.05). Amyloplasts were still covered almost entirely by the vacuolar membrane (Figure 4F). The effect of a microtubule-disrupting drug (amiprophosmethyl) on the movements of amyloplasts was further examined. The saltatory movements of amyloplasts were not affected by the treatment with amiprophosmethyl, and the stem showed normal gravitropic response (data not shown). components were also calculated (Table 1), and from this the value in zig-1/sgr4-1 was found to be noticeably reduced (0.46 mm/min, 0.19 mm/min, and 0.4 mm/min in 0 to 1 min, 1 to 2 min, and 2 to 3 min, respectively). The occurrence of the Dy positive components with large absolute values was not detected in zig-1/sgr4-1. DISCUSSION Dynamic Movement of Amyloplasts Is Severely Restricted in sgr2, sgr3, and zig/sgr4 Mutants Both before and after Gravistimuli Among sgr mutants, sgr2, sgr3, and zig/sgr4 were selected to observe the dynamics of amyloplasts in the endodermis of the inflorescence stem because these genes are likely to function in the biogenesis or the integrity of the vacuolar membrane (Kato et al., 2002; Yano et al., 2003). Electron microscopy studies have revealed that amyloplasts in the endodermis of the inflorescence stem of these sgr mutants did not sediment normally, nor were they wrapped by a vacuolar membrane (Morita et al., 2002; Yano et al., 2003). The movement of amyloplasts in these mutants is severely restricted in the endodermal cells of the inflorescence stem. Figures 5A and 5B show the behavior of amyloplasts in the endodermal cell of zig-1/sgr4-1 as a representative result from these mutants (see also Supplemental Movies 8 and 9 online). The amyloplasts in the endodermal cell of the mutants never moved through the central region of the cell, although they occasionally moved a very short distance in the peripheral area of the cell. To examine the defects of the vacuolar membrane in the endodermal cell, the GFP-g-TIP fusion construct was expressed in zig-1/sgr4-1 and the stems were subjected to microscopic observation. As a result, transvacuolar strand-like structures were not observed in the endodermal cell. In addition, many abnormal vesicle-like structures accumulated in these cells (Figure 5C), which occasionally showed very dynamic movement (see Supplemental Movie 10 online). Statistical analysis of the movement of amyloplasts in zig-1/sgr4-1 showed that before gravistimulation by reorientation, the distribution of Dy and Dx components became steeper, indicating that the movements of amyloplasts were inhibited (Figures 2D and 5D). After gravistimulation by reorientation, a small shift of Dy components to the positive occurred, but asymmetry of the distribution to the axis of x ¼ 0 was not significant. Neither mean values of Dx nor Dy in all time periods are statistically significantly increased compared with the mean values before gravistimulation by reorientation (Welch’s t test, one-tailed, P < 0.05). Average values of the Dy Subcellular Dynamics of the Endodermis in the Arabidopsis Inflorescence Stem In this study, the subcellular dynamics of the gravity-perceptive cell in the inflorescence stem of Arabidopsis were observed using fine time and image resolution, with several intriguing findings being noted. First, saltatory movement of amyloplasts was shown both before and after gravistimulation by reorientation in the living graviperceptive cell. Quantitative analysis of a large data set obtained from fine time-lapse imaging revealed that the amyloplasts actually moved toward the direction of gravity 0 to 3 min after gravistimulation by reorientation in the wild-type cell (Figure 2, Table 1). Saltatory movements of amyloplasts in the living graviperceptive cell have also been reported in the dandelion (Taraxacum officinale) flower stalks (Clifford and Barclay, 1980), mung bean (Vigna radiate) hypocotyls (Heathcote, 1981), and the maize (Zea mays) coleoptile (Sack and Leopold, 1985). Rapid movements of amyloplasts toward the gravity were detected (>3 mm/10 s, ;20 mm/min) as previously reported in the living graviperceptive cell; however, amyloplasts undergoing such movements constituted only a fraction of the overall population. Averaging the data sets of the amyloplast movement shows that gravity-oriented movement is attenuated by the upward movement component, although the movement can still be detected at ;1 mm/min. It is plausible that both kinds of gravity-oriented movement are actually occurring 0 to 3 min after gravistimulation by reorientation (Table 1, Figure 2D). These findings are consistent with the presentation time previously reported (Fukaki et al., 1996a). The vacuolar membrane was visualized and revealed that the translocation path of the amyloplasts occurs in transvacuolar strands, thin cytoplasmic areas that penetrate through the central vacuole. Sack and Leopold (1985) suggested that amyloplasts may move through transvacuolar strands. This study showed using confocal laser scanning microscopy that amyloplasts in the peripheral area of the cytoplasm were almost entirely covered with a vacuolar membrane. The vacuolar membrane itself seems to be dynamic, but it is continuously bent and deformed by Figure 4. (continued). (D) Top, time-lapse images of amyloplasts in the endodermal cell of latrunculin B–treated stem segment at 10-s intervals after gravistimulation by reorientation. Bottom, traces of amyloplasts. Each color represents the trace of an individual amyloplast. Bars ¼ 5 mm. (E) Histograms showing the frequency of the speed of amyloplasts tracks, extracted to Dx and Dy components in a wild-type stem segment treated with latrunculin B for 14 h. Total number subjected to the analysis before gravistimulation by reorientation (tracks n ¼ 404, cells n ¼ 3) and after gravistimulation by reorientation (tracks ¼ 520, cells n ¼ 4). (F) A confocal laser scanning microscopic image of the vacuolar membrane in the endodermal cell of a latrunculin B–treated stem segment. Bar ¼ 5 mm. Subcellular Dynamics of the Graviperceptive Cell 555 Figure 5. Impaired Dynamics of Both Amyloplasts and the Vacuolar Membrane in the zig-1/sgr4-1 Mutant Endodermal Cell. (A) Left, time-lapse images of amyloplasts at 1-min intervals before gravistimulation by reorientation. Right, trace of representative amyloplasts. Each color represents the trace of an individual amyloplast. Bars ¼ 5 mm. (B) Left, time-lapse images of amyloplasts at 1-min intervals after gravistimulation by reorientation. Right, trace of representative amyloplasts. Each color represents the trace of an individual amyloplasts. Bars ¼ 5 mm. (C) Time-lapse images of vacuolar membrane at 10-s intervals before gravistimulation by reorientation. Bar ¼ 5 mm. (D) Histograms showing the frequency of the speed of amyloplast tracks, extracted to Dx and Dy components in a zig-1/sgr4-1 stem segment. Total number subjected to the analysis before gravistimulation by reorientation (tracks n ¼ 869, cells n ¼ 3) and after gravistimulation by reorientation (tracks ¼ 490, n ¼ 3). the amyloplasts as well. As shown in Figures 1F and 3C, the amyloplasts are always closely attached and enwrapped by the vacuolar membrane. This interaction between vacuolar membrane and amyloplasts may represent one of the most striking differences between the subcellular organization of the root and shoot graviperceptive cells. In root collumera cells, amyloplasts are not enwrapped by the vacuolar membrane and are thought to move within the cytoplasm with little or no interaction with the vacuole. These findings are consistent with the observation that sgr2, sgr3, and zig/sgr4 mutants still have normal gravitropism in the root, in spite of their shoot gravitropism defects. Amyloplast Movement for Gravity Perception Is Likely to Be Actin Cytoskeleton Independent The actin cytoskeleton network has been characterized well in many plant species, with the thick actin cable often observed within the transvacuolar strands (Traas et al., 1987; McCurdy et al., 1988; Tominaga et al., 2000). This study also clearly showed the actin cytoskeleton in the transvacuolar strands and its involvement with the movement of amyloplasts. Findings from the actin disruption experiment suggest that the saltatory movement of amyloplasts through transvacuolar strands is dependent upon the actin cytoskeleton. 556 The Plant Cell The actin cytoskeleton has been proposed as a major player in plant gravitropism (Yoder et al., 2001; Blancaflor, 2002; Hou et al., 2003). Recent studies, however, showed that an intact actin cytoskeleton is not required for gravity perception in inflorescence stems and roots (Yamamoto and Kiss, 2002; Hou et al., 2003) and likely acts to downregulate gravitropism by continuously resetting the gravitropic-signaling system (Hou et al., 2004). The former finding was confirmed in this study, and in addition, the movement of amyloplasts was shown to correlate well with gravity-perceptive ability. In the actin disrupted cell, saltatory movements decline considerably both before and after gravistimulation by reorientation. After gravistimulation by reorientation, translocation of amyloplasts toward the gravity occurs, as shown by both time-lapse imaging and quantitative analysis (Figure 4, Table 1; see Supplemental Movies 6 and 7 online). A few of the amyloplasts can show dramatic and significant movement, even though most of the plastids do not move after reorientation. Although we cannot conclude from these observations that the movement of the amyloplasts was enhanced after actin disruption, as suggested by Yamamoto and Kiss (2002), we can say that the mass translocation of amyloplasts in the stem is comparable to the wild type. These results suggest that the movement of amyloplasts for graviperception is not actin dependent and that the saltatory movement of amyloplasts is actin dependent and not essential for graviperception. What Is the Importance of Movement of Amyloplasts for Graviperception? In this study, amyloplast dynamics were compared using three types of inflorescence stem: drug-untreated wild type, latrunculin B–treated wild type, and zig-1/sgr4-1 mutant. The former two are graviperceptive, and the latter is a nongraviperceptive stem. The results are summarized in Figure 6. These findings suggest two kinds of movement showing good correlation between gravity-perceptive ability. The first kind is the mass translocation of amyloplasts toward gravity. The average speed of the gravityoriented movement of amyloplasts in the latrunculin B–treated sample is comparable to that of the wild-type sample, whereas that of zig-1/sgr4-1 is greatly reduced (Table 1). These findings may indicate that mass translocation of amyloplasts toward the lower end of the cell induces gravity signal transduction and therefore is important for graviperception. The second kind of movement is displayed by a population of amyloplasts that sediment at high velocity (>3 mm/10 s; Figures 2 and 4, asterisks). That population is very small, a few percent, but they may retain a large amount of kinetic energy, taking into account that the kinetic energy is proportional to the square of the velocity, and this large amount of kinetic energy may be enough to overcome the threshold to elicit the signal for a downstream response. This kind of movement may correspond with the sedimentation of amyloplast ‘‘faster and to a greater magnitude,’’ as discussed by MacCleery and Kiss (1999) or ‘‘the leading amyloplasts’’ (Sack et al., 1984). The mean values of Dx direction in some time periods (in wild type, 2 to 3 and 3 to 4 min, and in the latrunculin B–treated samples 0 to 1 min and 3 to 4 min are reorientation) are also statistically significantly increased compared with the mean values before reorientation. The possible explanation for this Figure 6. Summary of This Work. observation is that amyloplasts tend to move toward the previous upper side of the cell because of the larger area than the previous bottom side of the cell. What Is the Role of the Complex Vacuolar Configuration—For Making Paths for Amyloplasts or Other Functions? The complex feature of the vacuole in the endodermal cell is presumed to have a function in gravitropism of the inflorescence stem. Because this close interaction of vacuolar membrane with amyloplasts was also reported in cress hypocotyls (Volkmann et al., 1993), this feature may be common in the shoot (in hypocotyls and inflorescence stems). Two possibilities of the function of vacuolar membrane in graviperception are proposed. First, it is proposed that the transvacuolar strands may facilitate smooth translocation of amyloplasts. In the wild type, amyloplasts are translocated within transvacuolar strands; however, in the zig/sgr4 mutant, amyloplasts cannot pass through the transvacuolar strands. In the latrunculin B–treated sample, the movement through transvacuolar strands is not clearly captured, but amyloplasts are still almost entirely enwrapped by a vacuolar membrane and therefore should pass thorough transvacuolar strands. The second proposal is that the vacuolar membrane itself may receive the signal directly from the gravity-oriented movement of amyloplasts, for example, through the change of tension in the membrane as a result of gliding amyloplasts. However, using this hypothesis, it is difficult to explain two factors. How is the noisy signal eliminated from continuously moving amyloplasts, and how do we know whether the signal coming from the vacuolar membrane within the cell is upward and/or downward. Because, at present, the vacuolar membrane itself seems not to have any particular polarity. Further careful study is required to address these questions. Subcellular Dynamics of the Graviperceptive Cell METHODS Sources and Constructions of GFP Marker Lines The GFP (S65T) with plastid transit peptide (Pt-GFP) of RBCS1A (Chiu et al., 1996; Niwa et al., 1999) was provided by Yasuo Niwa (Niwa et al., 1999; University of Shizuoka, Japan). A BamHI recognition sequence was inserted at 38 of RBCS1A. The BamHI-EcoRI fragment containing PtGFP and Nos terminator was then inserted into the BamHI and EcoRI sites of pBI121del_pSCR (Morita et al., 2002) downstream of ProSCR. The GFP (S65T) fused with g-tonoplast intrinsic protein (GFP-g-TIP) was provided by Hiroshi Abe (RIKEN, Wako, Saitama, Japan). The XbaI-EcoRI fragment containing GFP-g-TIP and Nos terminator was inserted into the XbaI and EcoRI sites of pBI121del_pSCR (Morita et al., 2002) downstream of ProSCR. The Pro35S:GFP-talin fusion construct was provided by Nam-Hai Chua (Kost et al., 1998; The Rockefeller University, New York, NY). The constructs were transformed into Agrobacterium tumefaciens strain MP90 by electroporation using the Gene pulser (Bio-Rad, Hercules, CA) and then introduced into wild-type Columbia plants using the floral dipping method (Clough and Bent, 1998). The T1 plants were selected with resistance to kanamycin. The construction of the transgene in these plants was tested using PCR. Segregation of the transgene in the T2 generation was confirmed. The representative lines were selected from T3 homozygous lines by checking the GFP fluorescence in the endodermis of the inflorescence stems. The transgenes were transferred to sgr mutants by transfection (GFP-g-TIP in zig-1/sgr4-1) or crossing (Pt-GFP in sgr2, sgr3, and zig-1/sgr4-1). Plant Materials and Growth Conditions In this study, pspt3-6 and psgt4-1 were used as a ProSCR:Pt-GFP and a ProSCR:GFP-g-TIP expressing line, respectively. Seeds were imbibed, sawed on MS medium (13 MS salt mixture, 1% [w/v] sucrose, 0.01% [w/ v] myoinositol, and 0.5% [w/v] gellan gum, pH 5.8) vernalized in the dark at 48C for 1 to 2 d, and then grown at 238C under continuous light. Seedlings were then transplanted to soil to grow inflorescence stems 7 to 14 d after germination. Only the first inflorescence stem was used in the experiments. Imaging System for Vertical Stage Microscope An epifluorescence microscope (BX-50; Olympus) equipped with rotary stage was mounted on its back so that the rotary stage was oriented vertically (Legue et al., 1997). Each endodermal cell in the inflorescence stem was observed while it was vertical, and its gravitropic response was elicited by rotating the stage through 908 until the cell layer was horizontal. The endodermal cell was observed before and after gravistimulation. GFP fluorescence or autofluorescence of amyloplasts was acquired with a cooled CCD camera (COOL SNAP cf; Photometrics, Nippon Roper, Tokyo, Japan) and processed using IPLab software (Scanalytics, Fairtax, VA). For confocal laser scanning microscopic observation, a confocal scanner unit (CSU-10; Yokogawa) was mounted to the vertical stage microscope. Images were captured by a CCD camera (ORCA-ER; Hamamatsu Photonics, Hamamatsu, Japan) and processed with Metamorph (Universal Imaging, Downingtown, PA) and IPLab. Sample Preparation for the Vertical Stage Microscope Pieces of cover glass (;170 mm thick; Matsunami, Tokyo, Japan) were cut into ;5-mm-wide strips. These strips were stuck to a glass slide as a spacer using a waterproof adhesive agent (ARON ALPHA; TOAGOSEI, Tokyo, Japan). Double-sided adhesive tape was prepared using two 557 sheets of an adhesive tape stuck together by ARON ALPHA. The doublesided adhesive tape was cut very thinly and stuck onto the spacer. Another piece of the double-sided adhesive tape was stuck inside the spacer, and an inflorescence stem segment (<1 cm in length, the region of 1 to 2 cm from the top of the inflorescence stem) was excised and stuck to the tape. The segment was cut longitudinally using a razor blade held with forceps. Immediately after cutting, a small amount of GM liquid medium (13 MS salt mixture and 1% sucrose) with 0.1% agar was added. The space inside the well was covered with a cover slip and sealed with double-sided adhesive tape (see Supplemental Figure 1 online). Electron Microscopy The samples of inflorescence stem were embedded in Spurr’s resin (Nisshin EM, Tokyo, Japan) following the methods of Morita et al. (2002). Ultrathin sections were cut (70 to 90 nm thick), stained with uranil acetate and lead nitrate, and examined with a transmission electron microscope (H-7100; Hitachi, Tokyo, Japan). Statistical Analysis of the Amyloplast Movement The movement of amyloplasts was subjected to quantitative analysis using time-lapse images. Amyloplasts that could be traced for a substantial period (approximately more than five frames) were selected. Each coordinate of the amyloplast in the image frame was recorded, and Dx and Dy was calculated at 10-s intervals. The axis of the coordinate was defined as shown in Figure 2. Pseudomovement caused by the directional and negligible stage drifting was corrected using the coordinate of an immobile marker (for example, the corner of the cell) in the first and last frames. Traces of the tracks were drawn using a representative example of each condition (before or after gravistimulation by reorientation in the wild type, zig-1/sgr4, and latrunculin B–treated stem segment). The frequency of Dx and Dy at each 10 s interval was presented in a histogram. Drug Treatment Stock solutions of latrunculin B (Calbiochem, La Jolla, CA; 20 mM) and amiprophosmethyl (Nihon Bayer Agrochem, Tokyo, Japan; 200 mM) were prepared in dimethyl sulfoxide. A stock solution of Cytochalasin B (Wako, Tokyo, Japan; 20 mM) was prepared in distilled water. First, drugs were added directly into the GM liquid medium. Latrunculin B and cytochalasin B effectively inhibited the saltatory movement of amyloplasts in the endodermal cell in 2 and 0.2 mM, respectively. The drug incorporation experiment was performed according to the methods of Fukaki et al. (1996a), with some modification. Distal 4-cm stem segments of primary inflorescence stems with total lengths between 4 and 12 cm were used for gravitropic response assays. The distal 4-cm parts were cut from the primary inflorescence stems with a razor blade, and their basal ends were inserted into a 1.5-mL microfuge tube positioned vertically on a tube rack. Several stem segments were soaked together in one tube so that they stood in a vertical position. They were preincubated for 14 to 16 h in white light at 238C in GM liquid medium without sucrose. After incubation, basal 5-mm pieces of these stem segments were put into GM gellan gum blocks that were fixed onto one face of plastic plates (Fukaki et al., 1996a). To maintain a high humidity in the plates, a wet paper towel was placed inside the plate. After the stem segments were incubated in a vertical orientation for 1 h under white light at 238C in the plates, gravistimulation was initiated by rotating the plates through 908 in darkness at 238C. Images were taken at the start point and after 150 min using the COOL SNAP cf camera. 558 The Plant Cell ACKNOWLEDGMENTS We thank Yasuo Niwa of the University of Shizuoka for the plantoptimized GFP clone, Hiroshi Abe of RIKEN for the GFP-gTIP fusion construct, Nam-Hai Chua for the GFP-talin fusion construct, and the members of the Tasaka laboratory for technical help and valuable comments on the manuscript. Especially, we would like to thank Seiko Ishihara for her excellent experimental assistance and Hidehiro Fukaki for his precious advice. We also thank Dean DellaPenna for enabling C.S. to accomplish part of this work in his laboratory. This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan, by a Special Coordination Fund for Promotion of Science and Technology from the Science and Technology Agency of Japan, by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists (C.S.; 03419), and by a grant from the Bioarchitect Project of RIKEN (M.T.M.). Received July 28, 2004; accepted November 19, 2004. REFERENCES Blancaflor, E.B. (2002). The cytoskeleton and gravitropism in higher plants. J. Plant Growth Regul. 21, 120–136. Chen, R., Rosen, E., and Masson, P.H. (1999). Gravitropism in higher plants. Plant Physiol. 120, 343–350. Chiu, W., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., and Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Curr. Biol. 6, 325–330. Clifford, P., and Barclay, G. (1980). The sedimentation of amyloplasts in living statocytes of the dandelion flower stalk. Plant Cell Environ. 3, 381–386. Clough, S., and Bent, A. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. Fasano, J.M., Swanson, S.J., Blancaflor, E.B., Dowd, P.E., Kao, T.-h., and Gilroy, S. (2001). Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13, 907–922. Fukaki, H., Diller, J.W., Kato, T., Fujisawa, H., Benfey, P.N., and Tasaka, M. (1998). Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 14, 425–430. Fukaki, H., Fujisawa, H., and Tasaka, M. (1996a). Gravitropic response of inflorescence stems in Arabidopsis thaliana. Plant Physiol. 110, 933–943. Fukaki, H., Fujisawa, H., and Tasaka, M. (1996b). How do plant shoots bend up? The initial step to elucidate the molecular mechanisms of shoot gravitropism using Arabidopsis thaliana. J. Plant Res. 109, 129–137. Heathcote, D. (1981). The geotropic reaction and statolith movements following geostimulation of mung bean hypocotyl. Plant Cell Environ. 4, 131–140. Hou, G., Kramer, V.L., Wang, Y.-S., Chen, R., Perbal, G., Gilroy, S., and Blancaflor, E.B. (2004). The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant J. 39, 113–125. Hou, G., Mohamalawari, D.R., and Blancaflor, E.B. (2003). Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiol. 131, 1360–1373. Johannes, E., Collings, D.A., Rink, J.C., and Allen, N.S. (2001). Cytoplasmic pH dynamics in maize pulvinal cells induced by gravity vector changes. Plant Physiol. 127, 119–130. Kato, T., Morita, M.T., Fukaki, H., Yamauchi, Y., Uehara, M., Niihama, M., and Tasaka, M. (2002). SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14, 33–46. Knight, T. (1806). On the direction of the radicle and germen during the vegetation of seeds. Philos. Trans. R. Soc. Lond. Biol. Sci. 99, 108–120. Kost, B., Spielhofer, P., and Chua, N. (1998). A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 16, 393–401. Legue, V., Blancaflor, E., Wymer, C., Perbal, G., Fantin, D., and Gilroy, S. (1997). Cytoplasmic free Ca2þ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 114, 789–800. MacCleery, S., and Kiss, J. (1999). Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol. 120, 183–192. Maurel, C., Reizer, J., Schroeder, J., and Chrispeels, M. (1993). The vacuolar membrane protein gamma-TIP creates water specific channels in Xenopus oocytes. EMBO J. 12, 2241–2247. McCurdy, D., Sammut, M., and Gunning, B. (1988). Immunofluorescent visualization of arrays of transverse cortical actin microfilaments in wheat root-tip cells. Protoplasma 147, 204–206. Morita, M.T., Kato, T., Nagafusa, K., Saito, C., Ueda, T., Nakano, A., and Tasaka, M. (2002). Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 14, 47–56. Niwa, Y., Hirano, T., Yoshimoto, K., Shimizu, M., and Kobayashi, H. (1999). Non-invasive quantitative detection and applications of nontoxic, S65T-type green fluorescent protein in living plants. Plant J. 18, 455–463. Sack, F. (1991). Plant gravity sensing. Int. Rev. Cytol. 127, 193–252. Sack, F., and Leopold, A. (1985). Cytoplasmic streaming affects gravity-induced amyloplast sedimentation in maize coleoptile. Planta 164, 56–62. Sack, F., Suyemoto, M.M., and Leopold, A.C. (1984). Kinetics of amyloplast sedimentation in gravistimulated maize coleoptiles. Planta 161, 459–464. Tasaka, M., Kato, T., and Fukaki, H. (1999). The endodermis and shoot gravitropism. Trends Plant Sci. 4, 103–107. Tominaga, M., Yokota, E., Vidali, L., Sonobe, S., Hepler, P.K., and Shimmen, T. (2000). The role of plant villin in the organization of the actin cytoskeleton, cytoplasmic streaming and the architecture of the transvacuolar strand in root hair cells of Hydrocharis. Planta 210, 836–843. Traas, J., Doonan, J., Rawlins, D., Shaw, P., Watts, J., and Lloyd, C. (1987). An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J. Cell Biol. 105, 387–395. Volkmann, D., Winn-Boerner, U., and Waberzeck, K. (1993). Graviresponsiveness of cress seedlings and structural status of presumptive statocytes from the hypocotyl. J. Plant Physiol. 142, 710–716. Weise, S., and Kiss, J. (1999). Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. Int. J. Plant Sci. 160, 521–527. Yamamoto, K., and Kiss, J.Z. (2002). Disruption of the actin cytoskeleton results in the promotion of gravitropism in inflorescence stems and hypocotyls of Arabidopsis. Plant Physiol. 128, 669–681. Yano, D., Sato, M., Saito, C., Sato, M.H., Morita, M.T., and Tasaka, M. (2003). A SNARE complex containing SGR3/AtVAM3 and ZIG/ VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc. Natl. Acad. Sci. USA 100, 8589–8594. Yoder, T.L., Zheng, H.-q., Todd, P., and Staehelin, L.A. (2001). Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol. 125, 1045–1060. Amyloplasts and Vacuolar Membrane Dynamics in the Living Graviperceptive Cell of the Arabidopsis Inflorescence Stem Chieko Saito, Miyo T. Morita, Takehide Kato and Masao Tasaka Plant Cell 2005;17;548-558 DOI 10.1105/tpc.104.026138 This information is current as of June 18, 2017 Supplemental Data /content/suppl/2005/01/14/tpc.104.026138.DC1.html References This article cites 33 articles, 13 of which can be accessed free at: /content/17/2/548.full.html#ref-list-1 Permissions https://www.copyright.com/ccc/openurl.do?sid=pd_hw1532298X&issn=1532298X&WT.mc_id=pd_hw1532298X eTOCs Sign up for eTOCs at: http://www.plantcell.org/cgi/alerts/ctmain CiteTrack Alerts Sign up for CiteTrack Alerts at: http://www.plantcell.org/cgi/alerts/ctmain Subscription Information Subscription Information for The Plant Cell and Plant Physiology is available at: http://www.aspb.org/publications/subscriptions.cfm © American Society of Plant Biologists ADVANCING THE SCIENCE OF PLANT BIOLOGY