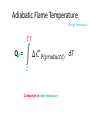* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Thermophotovoltaic wikipedia , lookup
Temperature wikipedia , lookup
Marcus theory wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Thermal conduction wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Heat transfer physics wikipedia , lookup
Transition state theory wikipedia , lookup
Thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Transcript
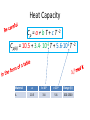
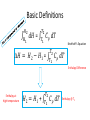
Thermo Dr. Nuri Solak, Asst. Prof. http://web.itu.edu.tr/solaknu/ www.ninova.itu.edu.tr http://web.itu.edu.tr/solaknu/ 1 11.09.2014 => Introduction, Definition of terms, Importance of thermodynamics in metallurgical and materials eng. 2 18.09.2014 => I. Law of thermodynamics, enthalpy, heat capacity, Kirchhoff equation 3 25.09.2014 => Heat of reaction, Hess Law, temperature dependency of heat of reaction 4 5 02.10.2014 => Combustion and fuel, adiabatic flame temperature 09.10.2014 => Adiabatic flame temperature 6 16.10.2014 => Combustion and fuel, adiabatic flame temperature, Energy balance 7 8 9 10 11 12 13 14 23.10.2014 => II. Law of thermodynamics, entropy, III. Law of thermodynamics, variation of entropy as a function of temperature 30.10.2014 => 1. Mid-term 06.11.2014 =>Standard free energy, equilibrium constant. 13.11.2014 => Calculation of composition of reaction under equilibrium conditions, Phase equilibrium in a one-component system 20.11.2014 => Standard free energy, equilibrium constant, calculation of composition of reaction under equilibrium conditions, Phase equilibrium in a one-component system 27.11.2014 => Standard free energy, equilibrium constant, calculation of composition of reaction under equilibrium conditions, Phase equilibrium in a one-component system 04.12.2014 => 2. Mid-term 11.12.2014 => Ellingham diagrams, Reduction reactions of oxides What is Thermodynamics • Thermodynamics is a science and, more importantly, an engineering tool used to describe processes that involve changes in temperature, transformation of energy, and the relationships between heat and work. • Thermodynamic is not only related to heat, it gives interrelation between different forms of energy. • Thermodynamics is derived from the Greek words therme, meaning heat, and dynamis, meaning power. • Thermodynamics is concerned only with the equilibrium state of matter. Basic Definitions A thermodynamic system (sistem) is a quantity of matter of fixed identity, around which we can draw a boundary. The boundaries may be fixed or moveable. Piston (boundary) and gas (system) Work or heat can be transferred across the system boundary. Everything outside the boundary is the surroundings (çevre). Basic Definitions Isolated system (Soyut ): “have walls or boundaries that are rigid, do not permit transfer of mechanical energy, perfectly insulating, and impermeable. They have a constant energy and mass content. Adiabatic systems (Adyabatik): Perfectly insulated systems. No energy transfer but matter can be transferred. Closed systems (kapalı): have walls that allow transfer of energy in or out of the system but are impervious to matter. They contain a fixed mass and composition, but variable energy. Open Systems (açık): have walls that allow transfer of both energy and matter to and from the system. Basic Definitions Homogenous System: A finite region in the physical system across which the physical and chemical properties are uniformly constant. It is also described as phase. Heterogeneous System: The system when it contains two or more phases, e.g., coexisting of ice and water. Extensive Materials Properties An extensive property is a physical quantity whose value is proportional to the size of the system it describes. • ENERGY !!! • entropy • enthalpy • mass • momentum • volume Instensive Materials Properties It is a physical property of a system that does not depend on the system size or the amount of material in the system • temperature • density (or specific gravity) • viscosity • electrical resistivity • hardness • melting point and boiling point • magnetization Macroscopic & Microscopic Energy • Potential energy and kinetic energy are macroscopic forms of energy. They can be visualized in terms of the position and the velocity of objects. • In addition to these macroscopic forms of energy, a substance possesses several microscopic forms of energy. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. It is so called internal energy due to atomic-molecular motion. E = U + KE + PE E = total energy U = Internal energy If we calculate change in energy dE = q – PdV - w (other, special conditions) Change in Energy (Produced work) Change in Thermodynamic Properties (Given Energy) dE = q – PdV d independent of the path taken (yoldan bağımsız) dependent of the path taken (yola bağımlı) If we heat up a system the given energy is not stored in the form of heat energy. It cause increase in internal energy, e.g., increase atomic motions, therefore temperature increases . 1. Law Thermodynamics dE = q – PdV ‘the change of a body inside an adiabatic system, from a given initial state to a given final state, involves the same amount of work by whatever means the process is carried out’ ‘Energy cannot be created, nor destroyed’ energy can change forms E = U + KE + PE E=U dE = dU = q – PdV Thermodynamic properties depend on : Temperature (T), Pressure (P), and Volume (V) T = f(P,V) E = f(P,V) 𝑑𝐸 = δ𝐸 δ𝑃 δ𝐸 𝑑𝑃 + δ𝑉 𝑉 P = f(T,V) E = f(T,V) V = f(T,P) E = f(T,P) 𝑑𝑉 𝑑𝐸 = 𝑃 𝑑𝐸 = δ𝐸 δ𝑇 δ𝐸 𝑑𝑇 + δ𝑉 𝑉 𝑑𝑉 𝑇 δ𝐸 δ𝑇 δ𝐸 𝑑𝑇 + δ𝑃 𝑃 𝑑𝑃 𝑇 In a closed system where E1 and V1 q amount of energy is given E2 and V2 are reached dE = dU = q - PdV E = E2 – E1 = qp – P (V2-V1) (E2 + PV2) – (E1 + PV1) = qp H2 H = E + PV Enthalpy H1 H2 – H1 = qp H = qp independent of the path dependent of the path Units • We use SI units! • Calorie, the quantity of heat required to raise the temperature of 1 gram of water from 14.5°C to 15.5°C. Now, we use Joule as energy unit, 1 cal = 4.18 Joule. • Temperature unit is Kelvin (K). • Gas constant R = 8.314 J/mol∙K Heat Capacity Heat capacity (C) of a system is the ratio of the heat added to or withdrawn from the system to resultant change in the temperature of the system. Heat capacity at constant volume is Cv and at constant pressure is Cp. δ𝑞 𝐶= 𝑑𝑇 (δq)p = (dH)p 𝐶𝑝 = δ𝑞 𝑑𝑇 δ𝑞 𝐶𝑝 = 𝑑𝑇 δ𝑞 𝐶𝑉 = 𝑑𝑇 𝑃 = 𝑃 𝑑𝐻 𝑑𝑇 𝑃 𝑉 Heat Capacity δ𝑞 𝐶𝑝 = 𝑑𝑇 𝐻2 𝑑𝐻 𝐻1 = = 𝑃 𝑑𝐻 𝑑𝑇 𝑇2 𝐶 𝑇1 𝑝 𝐻 = 𝐻2 − 𝐻1 = 𝑃 𝑑𝑇 𝑇2 𝐶 𝑇1 𝑝 𝑑𝑇 Kirchhoff’s Equation Required energy to increase temperature Cp = a + b T + cT -2 Heat Capacity • If there is a phase transition, e.g., melting of a metal, crystallographic (polymorphic) transition, then transition enthalpy should be included, like you did it at high school for melting latent energy (gizli ısı) for ice. • To indicate standart conditions ° sign is used. It is 1 atm and 25°C. Heat Capacity Cp = a + b T + c T -2 Cp(A) = 10.5 + 3.4∙ 10-3 T + 5.6∙105 T -2 Material A a b∙103 c∙10-5 Range (K) 10.5 3.4 5.6 200-2500 Heat Capacity 𝐻 = 𝐻2 − 𝐻1 = 𝑇2 𝐶 𝑇1 𝑝 Q = m C T Q = m.L Q = mC1 T + m.L + mC2 T 𝑑𝑇 Heat Capacity Values of Selected Materials @25°C [Cp (J K-1 g-1 or J oC-1 g-1)] Aluminium Cp = 0.90 Water Cp = 4.18 Carbon Cp = 0.72 Ethanol Cp= 2.44 Copper Cp = 0.39 H2SO4 Cp = 1.42 Lead Cp = 0.13 HCl Cp = 0.85 Mercury Cp= 0.14 KOH Cp= 1.18 What happened if the heat capacity of water were 0.4 instead 4 J/mol K Heat Capacity at low temperature Example - 1 1187K 1664K α-Fe γ-Fe -Fe 112 gram of α-Fe at 300K and γ–Fe at 1200K Calculate required energy to increase temperatere 100K for each case. Cp α-Fe = 37.12 + 6.17∙ 10-3 T (298 – 1187K) Cp γ-Fe = 24.48 + 8.45∙ 10-3 T (1187 – 1664K) Fe = 56 gr/mol a) 7855.9 J/112 gr b) 7008 J/112 gr Example - 2 at 27°C, take 112 gr Al2O3 In order to increase temperature 100°C, calculate the required energy. Cp Al2O3= 117.43 + 10.38∙ 10-3 T - 37.11 ∙ 105 T-2 Answer : 9903.74 J/112 gr (298 – 1800K) Hint: Be careful it is asked from 27°C to 127°C Actually, it means from 300K to 400 K Example - 3 Take 1 kg and 1 mol of ZrO2 and calculate required energy to increase temperature from: a) RT to 1200K 1450K α b) RT to 1600 K HT = 5900 J/mol Ttransition = 1450 K ZrO2 = 123.2 g/mol Cp α-ZrO2= 69.62 + 7.53∙ 10-3 T - 14.06 ∙ 105 T -2 Cp -ZrO2= 74.48 (1450 – 2800 K) (298 – 1450K) a) 521 781 J/kg b) 819 980 J/kg Homework • Plot Temp vs Cp for Al2O3 for the given temperature: T = 298, 350, 550, 600, 800, 950, 1100, 1500, 1550, 1650, 1800 K Cp Al2O3= 117.43 + 10.38∙ 10-3 T - 37.11 ∙ 105 T-2 Use Excel !!! (298 – 1800K) What are the advantages of high specific heat capacity of water? http://www.tutorvista.com/physics/animations /advantages-of-high-specific-heat-of-wateranimation What are the advantages of high specific heat capacity of water? http://www.tutorvista.com/physics/animations/ advantages-of-high-specific-heat-of-wateranimation Hess’s Law • Hess' law states that the energy change for any chemical or physical process is independent of the pathway or number of steps required to complete the process provided that the final and initial reaction conditions are the same. In other words, an energy change is path independent, only the initial and final states being of importance. (dH)p = (H)p Basic Definitions • Heat of reaction (H) (Reaksiyon Isısı) As a result of a reaction, the enthalpy difference between initial and final state. Released or absorbed energy of a chemical reaction. • H>0 Enthermic reaction, energy required • H<0 Exothermic reaction, energy release Basic Definitions • Enthalpy of Formation (Oluşum, Teşekkül) , it is a special form of heat of reaction. • H does not have an absolute value (only change in H can be measured). Therefore it is convenient to introduce a convention which allows the comparison of enthalpy difference. The convention assigns the value of zero to the enthalpy of elements in their stable states at 298K. Thus the enthalpy of compound at 298K is simply the heat of formation of compound from its elements. Basic Definitions 𝐻2 𝑑𝐻 𝐻1 = 𝑇2 𝐶 𝑇1 𝑝 𝐻 = 𝐻2 − 𝐻1 = 𝑑𝑇 Kirchhoff’s Equation 𝑇2 𝐶 𝑇1 𝑝 𝑑𝑇 Enthalpy Difference Enthalpy at high temperature 𝐻2 = 𝐻1 + 𝑇2 𝐶 𝑇1 𝑝 𝑑𝑇 Enthalpy @ T2 Basic Definitions A + B = C T = 298K Hess’s law 𝐻298 = 𝐻𝐶 298 − (α𝐻𝐴 298+ 𝐻𝐵 298) Important: This a chemical reaction, due to the reaction there is an Enthalpy Change ! A + B = C T=T 𝐻𝑇 = 𝐻𝐶 𝑇 − (𝐻𝐴 𝑇+ 𝐻𝐵 𝑇) Important: This a chemical reaction, due to the reaction there is an Enthalpy Change ! 𝐻𝐴 𝑇2 = 𝐻𝐴 𝑇1 + 𝑇2 𝐶 𝑇1 𝑝 𝑑𝑇 Fuels & Combustion • Combustion (Yanma) is used to describe exothermic combustion reaction of fuels. Oxidation reaction such as oxidation of aluminum is an exothermic reaction but it is not considered as combustion. • Conventional fuels can be in solid, liquid or gas state. The most commonly used fuels are hydrocarbons. • Reaction products are water vapour and CO2. Calorific Value (Kalorifik Güç) • The heating value or energy value of a substance, usually a fuel (or food), is the amount of heat released during the combustion of a specified amount of it. For solid and liquids it is 1 kg. For gases 1 m3. • Reaction is always exothermic but calorific value is taken positive. Adiabatic Flame Temperature (Alev Sıcaklığı) Fuel298 + Oxidation Agent298 = Reaction ProductsTF Fuel298 + Oxidation Agent298 = Q + Reaction Products298 Q + Reaction Products298 = Reaction ProductsTF Adiabatic Flame Temperature 𝑇𝑓 ∆𝐶′𝑃(𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠) dT Q298= 298 Combustion at room temperature Adiabatic Flame Temperature @ High Temperature 𝑇𝑓 ∆𝐶′𝑃(𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠) dT QT = 𝑇 Combustion at high temperature Flue gas (baca gazı) 𝑇𝑓𝑙𝑢𝑒 Qflue = 298 Always 298K ! Reference point ! ∆𝐶′𝑃(𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠) dT