* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download one way
Heat transfer physics wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Detailed balance wikipedia , lookup
Chemical imaging wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Thermodynamics wikipedia , lookup
Electrochemistry wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Ene reaction wikipedia , lookup
Rate equation wikipedia , lookup
Marcus theory wikipedia , lookup
Industrial catalysts wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Enzyme catalysis wikipedia , lookup
George S. Hammond wikipedia , lookup
Chemical potential wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
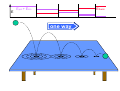
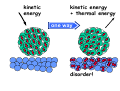
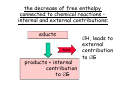
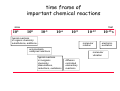
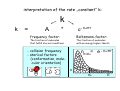
chemical reactions – two basic questions: + + 1) What is the driving force behind a chemical reaction? 2) How fast does a chemical reaction proceed? Chemical thermodynamics What drives a chemical reaction? The key parameter is the change of the Free Enthalpy ∆G general driving force for all spontaneous processes: the decrease of order, the decrease of free enthalpy ∆G Ordnung Unordnung one way ∆G < 0 Ordnung Unordnung decrease of order decrease of free enthalpy Epot + Ekin Etherm E one way kinetic energy kinetic energy + thermal energy one way disorder! the decrease of free enthalpy connected to chemical reactions internal and external contributions: educts heat products + internal contribution to ∆G ∆H, leads to external contribution to ∆G Chemical reactions primarily driven by the emission of heat: 2Cu + O2 2Na + Cl2 2CuO 2NaCl Chemical reactions primarily driven by the generation of internal disorder: H2CO3 NH4Cl H2O + CO2 HCl + NH3 Summary The key criterium is the decrease of the free enthalpy (or ∆G < 0 ). Two contributions to a negative enthalpy change are important: - the emission of heat - the generation of internal disorder Chemical kinetics how fast are chemical reactions? v = velocity of a chemical reaction The velocity of a given chemical reaction generally depends on three parameters: - the concentration of the educts - the temperature - the presence or the absence of a catalyst time frame of important chemical reactions slow 103 fast 100 10-3 10-6 10-9 typical reactions in organic chemistry: substitutions, additions... molecular rotation enzymatically catalyzed reactions typical reactions in inorganic chemistry: dissociations, reductions, oxidations 10-12 10-15 s electronic excitation molecular vibration diffusion controlled elementary reactions Chemical kinetics dependence of v on concentrations v generally depends on the concentration of the reaction educts A, B, C, ... (with concentrations cA cB cC ... ) according to the equation: v = k cAa cBb cCc ... with k being the rate constant of the reaction. The exponents a, b, c, ... characterize the individual dependencies of v on the concentrations of A, B, C, ... The sum s = a+b+c+... is called the order of the reaction. interpretation of the rate „constant“ k: k k = A * e - Ea/RT frequency factor: Boltzmann-factor: The fraction of molecules that fulfill sterical conditions The fraction of molecules with an energy higher than Ea - collision frequency - sterical factors (conformation, molecular orientation) nE>Ea= e - Ea /RT a n Ea E The Chemical Equilibrium example: v1 2A + B 2C + 3D v2 An equilibrium is reached, when both reaction rates are equal (v1 = v2). In this case, an equilibrium constant is defined as: c 2 c 3 Kc = C cA2 · D · cB Kc is constant at a given temperature. start: dice showing 5 or 6 points dice showing 6 points equilibrium: dice showing 5 or 6 points dice showing 6 points Summary The velocity v of a given chemical reaction generally depends on: - the concentration of the educts: according to v = k caA cbB ccC ... - the temperature: according to k = A exp(-Ea/RT) - the presence or the absence of a catalyst: a catalyst lowers the activation energy Ea The chemical equilibrium is reached when forward and backward reaction velocities are equal. In this case, an equilibrium constant Kc is given which is constant for a given temperature.