* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 112196 Primary Biliary Cirrhosis
Survey
Document related concepts
Behçet's disease wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Hepatitis B wikipedia , lookup
Signs and symptoms of Graves' disease wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Autoimmune encephalitis wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Hepatitis C wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Multiple sclerosis signs and symptoms wikipedia , lookup
Transcript
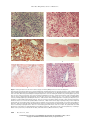
The New England Journal of Medicine Review Articles Medical Progress mation and allow determination of the histologic stage of disease, an important factor in assessing prognosis. P RIMARY B ILIARY C IRRHOSIS PREVALENCE, EPIDEMIOLOGY, AND GENETICS MARSHALL M. KAPLAN, M.D. Primary biliary cirrhosis affects members of all races and accounts for 0.6 to 2.0 percent of deaths from cirrhosis worldwide.2 Estimates of its prevalence range from 19 to 151 cases per million population, whereas estimates of its incidence range from 3.9 to 15 cases per million population per year.1,3-5 Genetic factors play a part in the development of the disorder. However, primary biliary cirrhosis is not inherited in any simple recessive or dominant pattern. Its prevalence in families with one affected member is estimated to be 1000 times higher than in the general population.6 Familial occurrences of the disease are found in sisters,7 brothers,8 brothers and sisters,9 and parent and child.10 Unaffected family members are more likely than normal persons to have impaired T-cell regulation11 and high levels of circulating autoantibodies, although significant increases in antimitochondrial antibodies are not found in healthy family members if purified recombinant mitochondrial autoantigens are used in the assay.12 There is a weak association between primary biliary cirrhosis and haplotype HLA-DR8,13,14 and in some populations there are associations with the DPB1 gene.15,16 P RIMARY biliary cirrhosis is a chronic, progressive cholestatic liver disease of unknown cause that usually affects middle-aged women and eventually leads to liver failure and the need for liver transplantation. It is diagnosed more frequently now than it was a decade ago because of its greater recognition by physicians and the widespread use of automated blood testing and the antimitochondrialantibody test, which is relatively specific for the disease.1 Important advances have been made in our understanding of the natural history, pathogenesis, and treatment of primary biliary cirrhosis since the subject was last reviewed in the Journal.2 Little has changed in its pathological features, diagnosis, and clinical manifestations. For completeness, however, these topics are also included here. Primary biliary cirrhosis is characterized by the destruction of small intraheptic bile ducts, portal inflammation, and progressive scarring. The typical patient is a middle-aged woman who reports fatigue and itching or who has no symptoms but has been found to have unexplained hepatomegaly or an elevated serum alkaline phosphatase concentration. Approximately 95 percent of patients are female. Biochemical tests of liver function typically reveal a cholestatic pattern — that is, the alkaline phosphatase and g-glutamyltransferase levels are disproportionately higher than the aminotransferase levels. Antimitochondrial-antibody tests are positive in 95 percent of patients. Demonstrating that the bile ducts are patent is important and can usually be done with ultrasonography. If the results of these tests are equivocal, computed tomographic (CT) scanning or endoscopic retrograde cholangiopancreatography should be performed. Percutaneous needle biopsy of the liver can provide confirmatory infor- From the Division of Gastroenterology, New England Medical Center, 750 Washington St., Boston, MA 02111, where reprint requests should be addressed to Dr. Kaplan. ©1996, Massachusetts Medical Society. 1570 PATHOLOGICAL FEATURES Gross Findings The liver in patients with primary biliary cirrhosis is characteristically enlarged and smooth, and it may be stained with bile. As the disease progresses, the liver may enlarge further, become finely nodular, and eventually appear grossly cirrhotic. The gallbladder is usually normal, although there is an increased prevalence of gallstones, by approximately 40 percent.17 Bile ducts are grossly normal. There is an increased prevalence of nodular regenerative hyperplasia in early-stage primary biliary cirrhosis. This may account for the unexpectedly high prevalence of portal hypertension and its complications in patients with clinically and histologically early stages of primary biliary cirrhosis.18 Enlarged lymph nodes are often seen in the porta hepatis, along the common bile duct, and less often in unexpected sites, such as the mesentery and supradiaphragmatic paracardiac areas.19 The enlargement is due to benign reactive hyperplasia.17 The spleen is normal early in the course of the disease, but it enlarges as hepatic fibro- Novem b er 2 1 , 1 9 9 6 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. MED ICA L PROGRES S sis and cirrhosis develop. Hypersplenism and evidence of portal hypertension are common in late stages. Hepatic Histology The course of primary biliary cirrhosis has been divided into four histologic stages.20,21 It is assumed that liver histology worsens and that the histologic stage advances over time from stage I to stage IV, frank cirrhosis. However, staging has inherent problems. Several stages, and occasionally all four, may be seen in a single biopsy specimen. The determination of stage is based on the most advanced lesion in the specimen. The liver may not be affected uniformly, and advanced lesions may be missed if the biopsy specimen is small. The initial lesion noted in biopsy specimens is damage to epithelial cells of the bile duct, presumably mediated by lymphocytes that surround and often infiltrate the duct. Epithelial cells may be vacuolated, shrunken, and pyknotic.20,21 Necrotic bile ducts are often located at the center of large granuloma-like lesions that consist of histiocytes, lymphocytes, plasma cells, eosinophils, and occasionally true giant cells21 (Fig. 1A). This is the florid bile-duct lesion in primary biliary cirrhosis. In this stage, inflammation remains confined to the portal triads. The lymphocytes that infiltrate the ductular epithelial cells directly and presumably destroy them are CD8 cells (suppressor–cytotoxic cells), but not natural killer cells.22 The majority of lymphocytes in the portal triads are CD4 cells (helper–inducer cells), although B cells are also seen.22,23 T cells cultured from triads appear to be clonal.24 Portal T cells express HLA class II antigens, interleukin-2 receptors, or both, and stain positively for tumor necrosis factor and interferon gamma, indicating that they are activated.25,26 Damaged bile ducts express intercellular adhesion molecule 1, vascular adhesion molecule, class II MHC molecules, and a molecule antigenically similar to the E2 subunit of mitochondrial pyruvate dehydrogenase.27,28 Granulomas are most often seen in stage I primary biliary cirrhosis. As the disease progresses to stage II, many portal triads become scarred, inflammatory cells spill out of the triads into the surrounding periportal parenchyma, normal bile ducts cut in cross section disappear, and atypical, poorly formed, tortuous bile ducts with no obvious lumens are seen (Fig. 1B). Periportal hepatocytes become vacuolated and surrounded by foamy macrophages, a process termed biliary piecemeal necrosis29 (Fig. 1C). In stage III, scarring progresses to the point at which fibrous septa link many adjoining portal triads (Fig. 1D). Stage IV is frank cirrhosis (Fig. 1E). Other histologic features of primary biliary cirrhosis are intracellular hyaline deposits in periportal areas identical to those seen in alcoholic liver dis- ease30 and increased amounts of stainable copper.31 The accumulation of copper correlates with the serum bilirubin level and with advancing stages of disease.32 IMMUNOLOGIC ABNORMALITIES Primary biliary cirrhosis has a sex distribution similar to that of other autoimmune diseases, with the great majority of patients being women. Abnormalities of both the humoral and cellular immune systems are common.33,34 The immunologic abnormalities seen in this disorder include increased levels of serum immunoglobulins, particularly IgM35,36; a failure to convert from IgM to IgG antibody after immunization37; the presence of a plethora of circulating autoantibodies; increased turnover of complement38; the presence of activated T cells and B cells in peripheral blood39; impaired T-cell regulation7; negative delayed-hypersensitivity skin tests; granulomas in the liver or lymph nodes draining the liver; and histologic liver lesions that resemble those occurring when hepatic allografts are rejected. There is an association between primary biliary cirrhosis and other diseases considered to have an autoimmune basis.40 Up to 84 percent of patients with primary biliary cirrhosis may have at least one other autoimmune disease, such as thyroiditis, scleroderma, rheumatoid arthritis, or Sjögren’s syndrome.40 Abnormalities of Humoral Immunity Some patients have a low-molecular-weight monomeric IgM in their blood and an apparent inability to synthesize the IgM pentamer.41 Others have increased serum levels of an IgM that is highly immunoreactive and highly cryoprecipitable.35 It spontaneously converts complement factor C3 to C3b and C3c through the classic pathway, behaves like an immune complex in some assays, and may lead to spuriously high serum IgM levels if the levels are measured by radioimmunodiffusion.42 Conversely, serum levels of IgE are decreased in some patients.43 Many circulating autoantibodies are found in patients with primary biliary cirrhosis, and the list is constantly growing. The most important diagnostically and perhaps pathogenetically is antimitochondrial antibody.44,45 Others include antinuclear antibodies, one of which is specific for nuclear membranes46; antithyroid antibodies47; lymphocytotoxic antibodies48; anti–acetylcholine-receptor antibodies49; antiplatelet antibodies50; anti–SS-A antibodies (also known as antiribonucleoprotein antigen Ro antibodies)51; antihistone antibodies52; and anticentromere antibodies.53 There is no increase in circulating immune complexes in patients with primary biliary cirrhosis.54 Earlier reports to that effect may reflect the presence of the abnormal IgM in the disease that causes spuriously positive results in some assays.35 Vol ume 335 Numbe r 21 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. 1571 The New England Journal of Medicine A B C D E F Figure 1. Histologic Features of the Liver in Various Stages of Primary Biliary Cirrhosis and of a Healthy Liver. Panel A shows an acute bile-duct injury in a patient with stage I primary biliary cirrhosis. The bile duct at the center is degenerating and is infiltrated by lymphocytes and fragments of lymphocytes. The epithelial cells are damaged and are missing from one quadrant of the duct, which has presumably been totally destroyed. The cells surrounding the bile duct are primarily lymphocytes, but there are also larger mononuclear cells and eosinophils (hematoxylin and eosin, 310). Panel B shows stage II primary biliary cirrhosis. There is atypical bile-duct hyperplasia. The bile ducts are tortuous, and few are cut in cross section. The inflammatory cells are primarily lymphocytes (hematoxylin and eosin, 310). Panel C shows foamy degeneration of hepatocytes adjacent to portal triads in a patient with primary biliary cirrhosis. There are collections of hyaline droplets in many of these swollen hepatocytes, identical to those seen in alcoholic hepatitis (Masson trichrome, 496). Panel D shows stage III primary biliary cirrhosis. Adjacent portal triads are connected by septa consisting of dense infiltrates of mononuclear cells and strands of connective tissue (Masson trichrome, 54). Panel E shows stage IV primary biliary cirrhosis. A wedge biopsy was obtained at the time of portacaval anastomosis for bleeding esophageal varices. There is a noncaseating granuloma in the center of a nodule. The portal triads are linked by bands of connective tissue and inflammatory cells (Masson trichrome, 90). Panel F shows a normal portal tract, containing a branch of the portal vein, an interlobular bile duct, and small arterioles (Masson trichrome, 250). 1572 Novem b er 2 1 , 1 9 9 6 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. MED ICA L PROGRES S Abnormalities of Cellular Immunity There are decreased numbers of circulating T lymphocytes (both helper and suppressor) in the blood of patients with primary biliary cirrhosis, as well as abnormalities in the regulation and function of these cells.55,56 Sequestration of T lymphocytes within hepatic portal triads probably accounts for their decreased numbers in the circulation.57 The decreased in vitro suppressor function of cells from some patients may be due to the abnormal activation and suppressor function of CD4 and Leu-8 T cells.58 Peripheral-blood T lymphocytes from patients with primary biliary cirrhosis do not respond normally to interleukin-2 and produce inadequate amounts of lymphotoxins, tumor necrosis factor, and interferon gamma when stimulated by mitogens.59,60 However, both circulating and intrahepatic T lymphocytes recognize and are stimulated by the E2 subunits of the mitochondrial oxo-acid dehydrogenase complexes61,62 and by a different bile-duct epithelial-cell antigen.63 Mitochondrial Antigens and Antibodies A major advance in our understanding of primary biliary cirrhosis occurred with the identification and cloning of the antigens against which antimitochondrial antibodies are detected.64 They are the dihydrolipoamide S-acetyltransferase component (E2 subunit) of a functionally related family of enzymes, the 2-oxo-acid dehydrogenases. These are pyruvate dehydrogenase, 3-methyl-2-oxobutanoate dehydrogenase (also known as branched-chain keto-acid dehydrogenase), and oxoglutarate dehydrogenase. Each may serve as an antigen for antimitochondrial antibodies, but pyruvate dehydrogenase is the most prevalent.45,65 Each catalyzes the reductive transfer of an acetyl group from its respective substrates to coenzyme A for oxidation in the Krebs cycle. Lipoic acid, a cofactor in the E2 subunit against which antimitochondrial antibodies are directed, is not an obligate component of the epitope. Human antimitochondrial antibodies clearly inhibit the enzymatic activity of these complexes in vitro.65 Thus far, all the mitochondrial autoantigens screened have been targets of only the anti-M2 antimitochondrial antibodies. Other antimitochondrial antibodies previously described, anti-M4, anti-M8, and anti-M9, are probably artifacts.66,67 Antimitochondrial antibodies are found in 95 percent of patients with primary biliary cirrhosis, and they have a specificity of 98 percent for this disease.45 Their role in the pathogenesis of the disorder is unclear. Their titers differ greatly among patients and do not correlate with the severity or rate of progression of disease.2 Antimitochondrial antibodies raised in animals immunized with recombinant human pyruvate dehydrogenase do not damage the bile duct or cause any recognizable disease.68 Human antimitochondrial antibodies recognize pyruvate dehydrogenase in Escherichia coli and yeast, but their binding affinities are several orders of magnitude lower than those of human antigens.69 Although the cause of primary biliary cirrhosis is still unknown, most data suggest that it is due to some inherited abnormality of immunoregulation whose precise nature is unknown.34 The fact that only one of two identical twins had the disease suggests that an additional factor, such as a triggering event that damages the epithelial cells of the bile duct, is also required in genetically susceptible persons.70 Potential triggers include treatment with interferon alfa and toxic effects on the liver from chlorpromazine, factors that may have initiated the development of primary biliary cirrhosis in two women.71,72 Such putative damage to bile-duct epithelial cells could then unmask a new antigen, such as pyruvate dehydrogenase complex E2, that is recognized by both hepatic and peripheral-blood T lymphocytes in primary biliary cirrhosis.61,62 A molecule that shares some antigenic determinants with the E2 subunit of pyruvate dehydrogenase is found on the luminal surface of biliary epithelial cells in patients with primary biliary cirrhosis early in their disease.28,73 Expression of this autoantigen on the luminal surface of biliary epithelial cells may provoke antibody-mediated attack by IgA antibodies, the antibodies present in bile.28 Alternatively, this E2-like antigen, together with the appropriate class II MHC molecules and another molecule required for antigen presentation, BB1/B7, could be the target of activated CD8 lymphocytes. All these molecules are found in and around damaged bile ducts in patients with primary biliary cirrhosis.73 The E2 component appears in damaged bile ducts before the class II MHC molecules and BB1/B7 appear. This sequence suggests that the E2 component may have a pathogenetic role. The histology of primary biliary cirrhosis is also consistent with T-lymphocyte–mediated cytotoxicity, as is the fact that the E2 antigens stimulate the production of interleukin-2 by cloned T cells isolated from liver tissue.60 In addition to the destruction of small bile ducts mediated by T lymphocytes, secondary damage to hepatocytes may result from the accumulation in the liver of increased concentrations of substances normally secreted into bile, such as bile acids. The foamy degeneration of hepatocytes in primary biliary cirrhosis has been attributed to the noxious effects of bile acids (Fig. 1C).29 Cholestasis in itself causes increased expression of HLA class I antigens on hepatocytes and renders them better targets for an immunologically mediated attack. Treatment with ursodiol reduces the expression of HLA class I antigens by hepatocytes, in addition to mitigating the Vol ume 335 Numbe r 21 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. 1573 The New England Journal of Medicine toxic effects of naturally occurring bile acids, such as cholic acid and chenodeoxycholic acid.74,75 CLINICAL FEATURES Symptoms and Physical Examination The onset of primary biliary cirrhosis typically occurs between the ages of 30 and 65, but I have seen and others have described women as young as 22 and as old as 93 at the time of diagnosis.76,77 Fatigue and pruritus are the usual presenting symptoms, with fatigue noted in up to 78 percent of patients.78 As many as 48 to 60 percent of patients may be asymptomatic.78,79 Pruritus may first occur during pregnancy and may be mistaken for the pruritus of pregnancy. However, the pruritus of pregnancy resolves in the postpartum period, whereas that due to primary biliary cirrhosis persists. Once pruritus occurs in a patient with biliary cirrhosis, it is unusual for the itching to disappear spontaneously. The same is true of jaundice. Itching is worse at night; under constricting, coarse garments; in association with dry skin; and in hot, humid weather. Pruritus is often not recognized as a sign of cholestasis, and many patients are referred to dermatologists. The cause of the pruritus in patients with primary biliary cirrhosis is unknown. It is not due to the retention of bile acids and their sequestration in skin80 but does respond to treatment with bile-acid–binding agents, such as cholestyramine resin. Increased opiodergic tone (that is, increased concentrations of endogenous opioid peptides and up-regulation of endogenous opioid receptors) related to chronic cholestasis has been suggested as a potential cause of the pruritus.81 Unexplained right-upper-quadrant discomfort was reported in 8 percent of patients in one study.82 In rare cases patients present with advanced disease that includes hemorrhage from esophageal varices, ascites, or hepatic encephalopathy.76,83 The findings on physical examination vary widely and depend on the stage of the disease at the time of presentation. The physical examination is often normal in asymptomatic patients. The skin is initially normal, but excoriations severe enough to cause bleeding may occur as the disease progresses. Xanthomas are a late manifestation. Striking hepatic enlargement is often found, occasionally in asymptomatic patients. Hepatomegaly becomes more common with progressive disease and is found eventually in approximately 70 percent of patients.77 Splenomegaly is present in 35 percent of patients at presentation but becomes more common as the disease progresses. Jaundice is a later manifestation of the disease, but in some patients it may be seen at presentation. Spider nevi, temporal and proximal-limb muscle wasting, ascites, and edema are all late manifestations of disease and suggest cirrhosis. Kayser– 1574 Fleischer rings are a very rare manifestation and result from the retention of copper.84 Laboratory Tests The serum alkaline phosphatase concentration is invariably elevated in patients with primary biliary cirrhosis, often to striking levels, and the enzyme is of hepatic origin. The level tends to reach a plateau early in the disease and usually fluctuates within 20 percent of that value.83 The serum levels of 5-nucleotidase and g-glutamyltransferase parallel those of alkaline phosphatase. The serum levels of alanine and aspartate aminotransferase may be normal or slightly elevated, rarely more than five times the upper limit of the normal value. They tend to fluctuate within a relatively narrow range and have no prognostic importance.85 The serum bilirubin level is usually normal early in the course of the disease, but it becomes elevated in 60 percent of patients as the disease progresses. Both the direct and indirect fractions are increased. An elevated serum bilirubin level is a sign of a poor prognosis.85 Serum lipids may be strikingly elevated in primary biliary cirrhosis. Serum cholesterol levels are elevated in at least half of patients76 and may exceed 1000 mg per deciliter in patients with xanthomas.86,87 Patients with early-stage primary biliary cirrhosis have mild elevations of low-density lipoprotein cholesterol and very-low-density lipoprotein cholesterol and marked elevations of high-density lipoprotein cholesterol.88 This may explain why patients with primary biliary cirrhosis have striking hypercholesterolemia but are not at increased risk for death from atherosclerosis.89 Another protective factor is their low serum levels of Lp(a) lipoprotein, an independent risk factor for coronary artery disease.90 Patients with advanced disease have striking elevations of low-density lipoprotein cholesterol, decreased levels of high-density lipoprotein cholesterol, and detectable levels of lipoprotein X, an abnormal lipoprotein seen in patients with chronic cholestasis.91 Other biochemical abnormalities include elevated serum ceruloplasmin levels, striking elevations of serum bile acids,92 and elevations of serum hyaluronate.93 Rising hyaluronate levels correlate with serum bilirubin levels and worsening histologic features.93 A previous report of impairment of sulfoxidation in primary biliary cirrhosis94 could not be confirmed.95 Diagnosis Primary biliary cirrhosis should be suspected in a patient who reports unexplained itching, fatigue, jaundice, or unexplained weight loss, with discomfort in the right upper quadrant and an unaccountable elevation of serum alkaline phosphatase. If the diagnosis is suspected, the patient should be questioned about symptoms frequently associated with it, such as dry eyes, dry mouth, arthritis, and Ray- Novem b er 2 1 , 1 9 9 6 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. MED ICA L PROGRES S naud’s phenomenon. It is important to question patients about the use of medications, because some drugs may cause cholestasis similar to that associated with primary biliary cirrhosis. If the alkaline phosphatase and serum IgM levels are both elevated and the antimitochondrial-antibody test is positive, primary biliary cirrhosis is likely. Slight increases in aminotransferases, to as much as four times the normal level, and elevations of serum cholesterol are corroborative data. The serum albumin level and the prothrombin time are typically normal at the time of diagnosis. Striking elevations of serum bile acids are characteristic, but this test is not commonly available. The diagnosis should be confirmed by a percutaneous liver biopsy, which will also provide information about the disease stage and the prognosis. The more portal triads there are in the specimen, the more likely it is that florid bile-duct lesions and granulomas will be found.21 If the history, physical findings, results of blood tests, and liver-biopsy findings are consistent, neither imaging nor cholangiography is needed. Associated Disorders Steatorrhea occurs primarily in patients with jaundice who have advanced disease. It is due to the decreased biliary secretion of bile acids and the resulting low concentration of bile acids in the small intestine — often lower than the critical micellar concentration.96,97 Pancreatic insufficiency may contribute to this condition.96,97 A diet low in neutral triglycerides and supplemented with medium-chain triglycerides and, when indicated, pancreatic extract will decrease the steatorrhea and improve nutritional status. The association of scleroderma, Sjögren’s syndrome, arthropathy, and the CREST syndrome (calcinosis cutis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) is well known.98,99 The scleroderma associated with primary biliary cirrhosis is rarely fatal.99 When specific testing for lacrimation was performed in one study, approximately 86 percent of patients had keratoconjunctivitis sicca, xerostomia, or both.100 Approximately 20 percent of patients have hypothyroidism,47 which may predate the onset of primary biliary cirrhosis or occur during its course. The prevalence of antithyroglobulin antibodies was 20 percent, and that of antimicrosomal antibodies 34 percent.47 Serum levels of thyroid-hormone–binding proteins are increased. The most accurate test for hypothyroidism is the test for serum thyroidstimulating hormone.101 Renal tubular acidosis occurs frequently but is usually subclinical.102 It is related to the deposition of copper in the kidney. About 40 percent of patients with primary biliary cirrhosis have defective urinary acidification after acid loading, but symptoms of acidosis are rare. Earlier reports of an increased prevalence of hepatocellular carcinoma and breast cancer in patients with primary biliary cirrhosis have not been confirmed in more recent, larger studies.103,104 I have also not found an increased prevalence of these cancers in 630 patients with primary biliary cirrhosis whom I have followed during the past 20 years. There is an increased prevalence of both asymptomatic bacteriuria and acute cystitis in patients with primary biliary cirrhosis.105 Antibiotic treatment appears to have little effect in asymptomatic patients. Bacteriuria spontaneously resolves in most asymptomatic patients, and eventually they become reinfected with different organisms. Osteoporosis is the bone disorder most often seen in patients with primary biliary cirrhosis.106 Osteomalacia occurs rarely. Symptomatic osteoporosis is seen less often, now that patients are referred for liver transplantation before the complications of longstanding cholestasis occur. The osteoporosis is not related to vitamin D deficiency,107 but its severity does correlate with the intensity and duration of jaundice,106 and unconjugated bilirubin inhibits the formation and function of osteoblasts in in vitro models of bone growth.108 There is no proved treatment for the osteoporosis other than liver transplantation, and improvement is first noted 6 to 12 months after surgery. Treatments with 25-hydroxyvitamin D plus calcium,106,109 ursodiol,110 and calcitonin111 have been ineffective. Fluoride (50 mg per day orally) was reported to prevent bone loss in seven patients followed for two years.112 Clinically important deficiencies of the fat-soluble vitamins A, K, and E are uncommon, except in patients with jaundice who have advanced primary biliary cirrhosis.113 Oral replacement therapy is usually adequate. Natural History and Prognosis Primary biliary cirrhosis progresses in most cases, but the rate of progression varies greatly among individual patients. Asymptomatic patients have substantially longer life expectancies than symptomatic ones, but their survival is still less than that of healthy patients matched for age and sex.78,114 The median survival of asymptomatic patients was 10 and 16 years in two large cohorts followed for up to 24 years.78,114 The median survival of symptomatic patients is approximately seven years.114,115 Symptoms develop in two to four years in most asymptomatic patients,78 but one third may remain symptom-free for many years.114 Neither the presence of antimitochondrial antibodies nor their titer affects survival. Some investigators have considered antimitochondrial-antibody–negative patients who have either antinuclear antibodies or antibodies to smooth muscle as having a separate disease, autoimmune cholangitis.116 However, these patients are identical to Vol ume 335 Numbe r 21 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. 1575 The New England Journal of Medicine patients with primary biliary cirrhosis in all other ways — for example, in histologic stage, results of biochemical tests, survival, and response to treatment.116 Prognosis and hence management decisions vary considerably depending on the clinical status of the patient. Whether to treat an asymptomatic patient is a difficult question. There is no reliable way to predict which such patients will have symptoms, nor is any therapy generally accepted as effective. If an asymptomatic patient has other diseases, such as thyroiditis, sicca syndrome, and scleroderma, survival is more likely to be decreased117 and cirrhosis more likely to be present.118 Granulomas are associated with better survival.119 The only laboratory measures of prognostic value — the serum bilirubin level, the serum albumin level, and the prothrombin time — are usually normal in asymptomatic patients. What is needed is a quantitative test of liver function that can identify asymptomatic patients who have decreased hepatic reserve. At the other end of the spectrum are patients with clinically advanced disease. Here, the major consideration is the timing of liver transplantation. There are more than five prognostic models for predicting survival, most based on Cox multiple-regression analysis.118-123 The only variable common to all five is the serum bilirubin level. These models are helpful in the timing of liver transplantation, but they are useful only for patients with advanced disease. All depend on variables that are primarily manifestations of the failing liver — namely, elevated serum bilirubin levels, decreased serum albumin levels, prolonged prothrombin time, fluid retention, and hemorrhage from esophageal varices. Patients with any of these negative prognostic indicators probably have both cirrhosis and irreversible, medically untreatable disease. Even if medical treatment is attempted in such patients, they should be evaluated for liver transplantation. TREATMENT OF SYMPTOMS The most common symptom that is relatively specific for primary biliary cirrhosis is pruritus. Cholestyramine resin (4 g three times per day orally) will relieve pruritus in most patients. The dose of this nonabsorbed resin must be adjusted in individual patients, and it takes one to four days from the initiation of treatment for the itching to remit. Antihistamines are helpful only early in the course of the disease, when itching is not severe. Another ammonium resin, colestipol hydrochloride, is as effective as cholestyramine. Rifampin,120 ursodiol,121 and naloxone81 control itching in some patients who are unresponsive to cholestyramine. Large-volume plasmapheresis relieves the symptom in the rare patient who does not respond to any of these medications.122 Individual patients have responded to pho1576 totherapy with ultraviolet B light, methyltestosterone, cimetidine, phenobarbital, and prednisone. The complications of primary biliary cirrhosis, except for symptomatic osteoporosis and hemorrhage from esophageal varices in patients with early-stage disease, are similar to those in patients with other types of cirrhosis and are not reviewed here. The management of osteoporosis has already been discussed. Unlike patients with other types of cirrhosis, those with primary biliary cirrhosis may have bleeding from esophageal varices early in the course of the disease, before there is jaundice123 or true cirrhosis.124 Distal splenorenal shunting is the preferred treatment.125 Survival is not adversely affected and is similar to that predicted by the Mayo prognostic model.123,125,126 TREATMENT OF UNDERLYING DISEASE There is no generally accepted treatment for the underlying disease process in primary biliary cirrhosis, but the results with ursodiol, colchicine, and methotrexate are encouraging. Glucocorticoids do not appear to improve the course of the disease and may worsen osteoporosis. In one prospective trial of low-dose prednisolone, the only significant effect after three years was a decrease in serum immunoglobulins and alkaline phosphatase activity.127 Azathioprine has limited efficacy and is no longer used.118 Penicillamine, an agent that induces cupriuria and has some antiinflammatory actions, is ineffective and caused side effects in more than 25 percent of patients in one study.115 Ursodiol Ursodiol has been evaluated in four randomized, prospective, double-blind trials.121,128-130 The appropriate dose is 12 to 15 mg per kilogram of body weight per day, given either in divided doses or as one dose at bedtime. It is safe and well tolerated. Diarrhea occurs in less than 2 percent of patients. Ursodiol lowers the serum levels of bilirubin, alkaline phosphatase, g-glutamyltransferase, alanine and aspartate aminotransferase, and IgM. It relieves pruritus in some patients, although it may exacerbate that symptom during the first two weeks of treatment. Its effect on liver histology is uncertain. Ursodiol decreased hepatic inflammation in one study,121 but not in the others.128-130 Ursodiol extended the time before a patient died or was referred for liver transplantation, as compared with placebo, in studies that continued for four years.121 When the data from three of these studies were combined and analyzed, ursodiol slightly prolonged the time before liver transplantation was indicated, as compared with placebo (3.66 vs. 3.45 years, P0.014), and decreased the likelihood of liver transplantation or death by 32 percent.131 Ursodiol appears to be more effective in patients with Novem b er 2 1 , 1 9 9 6 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. MED ICA L PROGRES S early disease than in those with later disease stages.132 In one trial, the results of biochemical tests became completely normal in 12 of 65 patients after two years of treatment. As compared with the other patients, these 12 patients had less advanced histologic stages of disease and lesser elevations of serum bilirubin and alkaline phosphatase at the time that ursodiol was given. Ursodiol is ineffective in patients with advanced disease and may worsen symptoms and blood-test results in some patients.73 Because ursodiol may lower serum bilirubin levels in patients with clinically and histologically progressive disease, treatment with the drug may take away the predictive value of bilirubin in the timing of liver transplantation.93 Cyclosporine Cyclosporine has been evaluated prospectively in two short-term double-blind studies and one sixyear study.133,134 In 29 patients with precirrhotic primary biliary cirrhosis, cyclosporine (4 mg per kilogram per day orally) stabilized fatigue and itching and decreased serum levels of bilirubin, alanine aminotransferase, alkaline phosphatase, gamma globulin, and antimitochondrial antibody.133 Most patients had hypertension and renal toxic effects. In the largest treatment trial to date, 349 patients were randomly assigned to receive 3 mg per kilogram per day of cyclosporine or placebo and were followed for up to six years.134 The cyclosporine-treated patients had slight but significant improvements in levels of albumin, alkaline phosphatase, serum bilirubin, and alanine aminotransferase, but worsening in serum creatinine levels. Survival was not improved except when a Cox proportional-hazards model was used to correct for the fact that the patients who received cyclosporine were sicker. Pruritus was improved, but not fatigue or histology. The limited efficacy of cyclosporine is counterbalanced by its predictable toxicity. Colchicine Colchicine (0.6 mg twice daily) significantly improved serum levels of bilirubin, albumin, alkaline phosphatase, cholesterol, and the aminotransferases after two years of follow-up and significantly improved survival after four years in one prospective, double-blind trial.135 There was lesser improvement in these variables in two similar studies.136,137 Histology and symptoms did not improve in any of these trials. Patients in one study were followed for up to eight years, at which time the results of biochemical tests were stable but there was no survival benefit.138 One placebo-controlled trial of 90 patients compared colchicine with ursodiol.139 Both drugs improved pruritus. Colchicine improved the biochemical values slightly, whereas ursodiol reduced serum alkaline phosphatase and aminotransferase activities significantly as compared with placebo or colchicine and also lowered serum bilirubin levels. Ursodiol and colchicine were synergistic when they were used in combination in 12 patients followed for two years.140 Methotrexate Methotrexate (15 mg per week orally) decreased serum levels of alkaline phosphatase, alanine and aspartate aminotransferase, cholesterol, and bilirubin in a pilot study of nine patients followed for two years.141 All the patients had improvement or resolution of their fatigue, their itching, or both. Most patients had transient increases in aminotransferase levels two to eight weeks after treatment with methotrexate began, followed by decreases in the levels and often a return to normal values. Serum biochemical values became normal in five of these patients with prolonged treatment (60 months), and their mean histologic stage decreased from 2.5 to 1.0.142 Methotrexate did not benefit patients with advanced cirrhosis or decompensated liver disease.141 The response to methotrexate is slower than the response to ursodiol, and improvements in biochemical values with methotrexate may continue slowly for up to four years.142 In an interim (24 month) analysis of a randomized, double-blind trial comparing methotrexate with colchicine in 89 patients, enzyme measurements improved significantly in both groups, but the decrease in alkaline phosphatase and the aminotransferases was significantly greater with methotrexate.143 Pruritus and liver histology improved significantly in the methotrexate group.143,144 In both treatment groups, the synthesis of interleukin-1b by cultured peripheral-blood monocytes increased significantly over the two-year period.145 The effects of methotrexate and ursodiol appear to be additive.146 Methotrexate improved symptoms and biochemical values in one study of eight patients who had only partial responses to ursodiol.147 However, in another study of 32 patients who received methotrexate and ursodiol together, no additive effect was observed when these patients were compared with patients from an earlier study of ursodiol alone.148 Interstitial pneumonitis is a serious problem in patients with primary biliary cirrhosis who are treated with methotrexate.149 That condition occurred in 14 percent of patients in one study but responded promptly when methotrexate therapy was discontinued and glucocorticoids were instituted. Liver Transplantation The only treatment that clearly improves the natural history of primary biliary cirrhosis is liver transplantation.150 There is 85 to 90 percent survival at one year, and survival rates thereafter resemble those Vol ume 335 Numbe r 21 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. 1577 The New England Journal of Medicine of healthy persons matched for age and sex. For all practical purposes, primary biliary cirrhosis does not recur after liver transplantation if appropriate immunosuppression is used.151 However, a minority of patients may have minor histologic lesions that resemble those in early primary biliary cirrhosis, although the patients are asymptomatic and have normal biochemical values.152 There are close clinical and histologic similarities between patients with primary biliary cirrhosis and patients in whom transplanted livers are rejected. Regimens in which immunosuppressive agents are combined effectively prevent such rejection. Therefore, future treatments for primary biliary cirrhosis will probably use combinations of drugs.153 I am indebted to Jane Bankoff-Popkin and Drs. Andrew Leiter and Arthur Rabson for their assistance in the preparation of the manuscript. REFERENCES 1. Myszor M, James OF. The epidemiology of primary biliary cirrhosis in north-east England: an increasingly common disease? Q J Med 1990;75: 377-85. 2. Kaplan MM. Primary biliary cirrhosis. N Engl J Med 1987;316:521-8. 3. Watson RG, Angus PW, Dewar M, Goss B, Sewell RB, Smallwood RA. Low prevalence of primary biliary cirrhosis in Victoria, Australia. Gut 1995;36:927-30. 4. Danielsson A, Boqvist L, Uddenfeldt P. Epidemiology of primary biliary cirrhosis in a defined rural population in the northern part of Sweden. Hepatology 1990;11:458-64. 5. Villeneuve J-P, Fenyves D, Infante-Rivard C. Descriptive epidemiology of primary biliary cirrhosis in the province of Quebec. Clin Gastroenterol 1991;5:174-8. 6. Bach N, Schaffner F. Familial primary biliary cirrhosis. J Hepatol 1994; 20:698-701. 7. James SP, Jones EA, Schafer DF, Hoofnagle JH, Varma RR, Strober W. Selective immunoglobulin A deficiency associated with primary biliary cirrhosis in a family with liver disease. Gastroenterology 1986;90:283-8. 8. Galbraith RM, Smith M, Mackenzie RM, Tee DE, Doniach D, Williams R. High prevalence of seroimmunologic abnormalities in relatives of patients with active chronic hepatitis or primary biliary cirrhosis. N Engl J Med 1974;290:63-9. 9. Schaffner F. Primary biliary cirrhosis. Clin Gastroenterol 1975;4:35166. 10. Tong MJ, Nies KM, Reynolds TB, Quismorio FP. Immunological studies in familial primary biliary cirrhosis. Gastroenterology 1976;71:3057. 11. Miller KB, Sepersky RA, Brown KM, Goldberg MJ, Kaplan MM. Genetic abnormalities of immunoregulation in primary biliary cirrhosis. Am J Med 1983;75:75-80. 12. Caldwell SH, Leung PS, Spivey JR, et al. Antimitochondrial antibodies in kindreds of patients with primary biliary cirrhosis: antimitochondrial antibodies are unique to clinical disease and are absent in asymptomatic family members. Hepatology 1992;16:899-905. 13. Underhill J, Donaldson P, Bray G, Doherty D, Portmann B, Williams R. Susceptibility to primary biliary cirrhosis is associated with the HLADR8-DQB1*0402 haplotype. Hepatology 1992;16:1404-8. 14. Gregory WL, Mehal W, Dunn AN, et al. Primary biliary cirrhosis: contribution of HLA class II allele DR8. Q J Med 1993;86:393-9. 15. Mella JG, Roschmann E, Maier KP, Volk BA. Association of primary biliary cirrhosis with the allele HLA-DPB1*0301 in a German population. Hepatology 1995;21:398-402. 16. Underhill JA, Donaldson PT, Doherty DG, Manabe K, Williams R. HLA DPB polymorphism in primary sclerosing cholangitis and primary biliary cirrhosis. Hepatology 1995;21:959-62. 17. Summerfield JA, Elias E, Hungerford GD, Nikapota VL, Dick R, Sherlock S. The biliary system in primary biliary cirrhosis: a study by endoscopic retrograde cholangiopancreatography. Gastroenterology 1976;70:240-3. 18. Colina F, Pinedo F, Solis JA, Moreno D, Nevado M. Nodular regen- 1578 erative hyperplasia of the liver in early histological stages of primary biliary cirrhosis. Gastroenterology 1992;102:1319-24. 19. Outwater E, Kaplan MM, Bankoff MS. Lymphadenopathy in primary biliary cirrhosis: CT observations. Radiology 1989;171:731-3. 20. Scheuer P. Primary biliary cirrhosis. Proc R Soc Med 1967;60:125760. 21. Ludwig J. New concepts in biliary cirrhosis. Semin Liver Dis 1987;7: 293-301. 22. Hashimoto E, Lindor KD, Homburger HA, et al. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clin Proc 1993;68:1049-55. 23. Nakanuma Y. Distribution of B lymphocytes in nonsuppurative cholangitis in primary biliary cirrhosis. Hepatology 1993;18:570-5. 24. Moebius U, Manns M, Hess G, Kober G, Meyer zum Buschenfelde KH, Meuer SC. T cell receptor gene rearrangements of T lymphocytes infiltrating the liver in chronic active hepatitis B and primary biliary cirrhosis (PBC): oligoclonality of PBC-derived T cell clones. Eur J Immunol 1990; 20:889-96. 25. MacSween RN, Burt AD. The cellular pathology of primary biliary cirrhosis. Mol Aspects Med 1985;8:269-91. 26. Colucci G, Schaffner F, Paronetto F. In situ characterization of the cellsurface antigens of the mononuclear cell infiltrate and bile duct epithelium in primary biliary cirrhosis. Clin Immunol Immunopathol 1986;41:35-42. 27. Yasoshima M, Nakanuma Y, Tsuneyama K, Van de Water J, Gershwin ME. Immunohistochemical analysis of adhesion molecules in the microenvironment of portal tracts in relation to aberrant expression of PDC-E2 and HLA-DR on the bile ducts in primary biliary cirrhosis. J Pathol 1995; 175:319-25. 28. Van de Water J, Turchany J, Leung PS, et al. Molecular mimicry in primary biliary cirrhosis: evidence for biliary epithelial expression of a molecule cross-reactive with pyruvate dehydrogenase complex-E2. J Clin Invest 1993;91:2653-64. 29. Portmann B, Popper H, Neuberger J, Williams R. Sequential and diagnostic features in primary biliary cirrhosis based on serial histologic study in 209 patients. Gastroenterology 1985;88:1777-90. 30. MacSween RN. Mallory’s (‘alcoholic’) hyaline in primary biliary cirrhosis. J Clin Pathol 1973;26:340-2. 31. Vaux DJ, Watt F, Grime GW, Takacs J. Hepatic copper distribution in primary biliary cirrhosis shown by the scanning protein microprobe. J Clin Pathol 1985;38:653-8. 32. Kowdley KV, Knox TA, Kaplan MM. Hepatic copper content is normal in early primary biliary cirrhosis and primary sclerosing cholangitis. Dig Dis Sci 1994;39:2416-20. 33. Gershwin ME, Mackay IR. Primary biliary cirrhosis: paradigm or paradox for autoimmunity. Gastroenterology 1991;100:822-33. 34. James SP, Hoofnagle JH, Strober W, Jones EA. Primary biliary cirrhosis: a model autoimmune disease. Ann Intern Med 1983;99:500-12. 35. Lindgren S, Eriksson S. IgM in primary biliary cirrhosis: physicochemical and complement activating properties. J Lab Clin Med 1982;99:636-45. 36. Nouri-Aria KT, Hegarty JE, Neuberger J, Eddleston AL, Williams R. In vitro studies on the mechanism of increased serum IgM levels in primary biliary cirrhosis. Clin Exp Immunol 1985;61:297-304. 37. Thomas HC, Holden R, Jones JV, Peacock DB. Immune response to phi X 174 in man. 5. Primary and secondary antibody production in primary biliary cirrhosis. Gut 1976;17:844-8. 38. Lindgren S, Johnson U. Complement activation in primary biliary cirrhosis: an in vitro model. J Lab Clin Med 1985;105:432-5. 39. James SP, Jones EA, Hoofnagle JH, Strober W. Circulating activated B cells in primary biliary cirrhosis. J Clin Immunol 1985;5:254-60. 40. Culp KS, Fleming CR, Duffy J, Baldus WP, Dickson ER. Autoimmune associations in primary biliary cirrhosis. Mayo Clin Proc 1982;57:365-70. 41. Roberts-Thomson PJ, Shepherd K. Low molecular weight IgM in primary biliary cirrhosis. Gut 1990;31:88-91. 42. Fakunle YM, Aranguibel F, de Villiers D, Thomas HC, Sherlock S. Monomeric (7S) IgM in chronic liver disease. Clin Exp Immunol 1979;38: 204-10. 43. Minuk GY, Boyd ND, Matheson DS, Fritzler MJ, Green BJ. Serum immunoglobulin E levels in patients with primary biliary cirrhosis. J Allergy Clin Immunol 1989;83:462-6. 44. Walker JG, Doniach D, Roitt IM, Sherlock S. Serological tests in diagnosis of primary biliary cirrhosis. Lancet 1965;1:827-31. 45. Van de Water J, Cooper A, Surh CD, et al. Detection of autoantibodies to recombinant mitochondrial proteins in patients with primary biliary cirrhosis. N Engl J Med 1989;320:1377-80. 46. Wesierska-Gadek J, Hohenauer H, Hitchman E, Penner E. Autoantibodies from patients with primary biliary cirrhosis preferentially react with the amino-terminal domain of nuclear pore complex glycoprotein gp210. J Exp Med 1995;182:1159-62. Novem b er 2 1 , 1 9 9 6 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. MED ICA L PROGRES S 47. Elta GH, Sepersky RA, Goldberg MJ, Connors CM, Miller KB, Kaplan MM. Increased incidence of hypothyroidism in primary biliary cirrhosis. Dig Dis Sci 1983;28:971-5. 48. Pares A, Martorell J, Caballeria J, Vives J, Bruguera M, Rodes J. Lymphocytotoxic antibodies in primary biliary cirrhosis. Dig Dis Sci 1985;30: 829-33. 49. Sundewall AC, Lefvert AK, Olsson R. Anti-acetylcholine receptor antibodies in primary biliary cirrhosis. Acta Med Scand 1985;217:519-25. 50. Bassendine MF, Collins JD, Stephenson J, Saunders P, James OF. Platelet associated immunoglobulins in primary biliary cirrhosis: a cause of thrombocytopenia? Gut 1985;26:1074-9. 51. Penner E. Demonstration of immune complexes containing the ribonucleoprotein antigen Ro in primary biliary cirrhosis. Gastroenterology 1986;90:724-7. 52. Penner E, Muller S, Zimmermann D, Van Regenmortel MH. High prevalence of antibodies to histones among patients with primary biliary cirrhosis. Clin Exp Immunol 1987;70:47-52. 53. Parveen S, Morshed SA, Nishioka M. High prevalence of antibodies to recombinant CENP-B in primary biliary cirrhosis: nuclear immunofluorescence patterns and ELISA reactivities. J Gastroenterol Hepatol 1995;10: 438-45. 54. Goldberg MJ, Kaplan MM, Mitamura T, et al. Evidence against an immune complex pathogenesis of primary biliary cirrhosis. Gastroenterology 1982;83:677-83. 55. Miller KB, Elta GH, Rudders RA, Kaplan MM. Lymphocyte subsets in primary biliary cirrhosis. Ann Intern Med 1984;100:385-7. 56. James SP, Elson CO, Waggoner JG, Jones EA, Strober W. Deficiency of the autologous mixed lymphocyte reaction in patients with primary biliary cirrhosis. J Clin Invest 1980;66:1305-10. 57. Moreno-Otero R, Civeira MP, Suou T, Kanof ME, James SP, Jones EA. Reduced numbers of CD8 T cells and B cell-expression of Leu-8 antigen in peripheral blood of patients with primary biliary cirrhosis. Hepatogastroenterology 1994;41:239-43. 58. Suou T, Civeira MP, Kanof ME, Moreno-Otero R, Jones EA, James SP. Defective immunoregulation in primary biliary cirrhosis: CD4, Leu-8 T cells have abnormal activation and suppressor function in vitro. Hepatology 1989;10:408-13. 59. Menendez JL, Giron JA, Manzano L, et al. Deficient interleukin-2 responsiveness of T lymphocytes from patients with primary biliary cirrhosis. Hepatology 1992;16:931-6. 60. Spengler U, Moller A, Jung MC, et al. T lymphocytes from patients with primary biliary cirrhosis produce reduced amounts of lymphotoxins, tumor necrosis factor and interferon-gamma upon mitogen stimulation. J Hepatol 1992;15:129-35. 61. Van de Water J, Ansari AA, Surh CD, et al. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J Immunol 1991;146:89-94. 62. Jones DE, Palmer JM, James OF, Yeaman SJ, Bassendine MF, Diamond AG. T-cell responses to the components of pyruvate dehydrogenase complex in primary biliary cirrhosis. Hepatology 1995;21:995-1002. 63. Miyamoto T, Maeda T, Onishi S, Yamamoto Y. T cell autoreactivity against a 28 kD biliary protein (B1-p28) in primary biliary cirrhosis. J Hepatol 1995;22:423-30. 64. Coppel RL, McNeilage LJ, Surh CD, et al. Primary structure of the human M2 mitochondrial autoantigen of primary biliary cirrhosis: dihydrolipoamide acetyltransferase. Proc Natl Acad Sci U S A 1988;85:731721. 65. Van de Water J, Fregeau D, Davis P, et al. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. J Immunol 1988;141:2321-4. 66. Davis PA, Leung P, Manns M, et al. M4 and M9 antibodies in the overlap syndrome of primary biliary cirrhosis and chronic active hepatitis: epitopes or epiphenomena? Hepatology 1992;16:1128-36. 67. Palmer JM, Yeaman SJ, Bassendine MF, James OF. M4 and M9 autoantigens in primary biliary cirrhosis — a negative study. J Hepatol 1993;18: 251-4. 68. Krams SM, Surh CD, Coppel RL, Ansari A, Ruebner B, Gershwin ME. Immunization of experimental animals with dihydrolipoamide acetyltransferase, as a purified recombinant polypeptide, generates mitochondrial antibodies but not primary biliary cirrhosis. Hepatology 1989;9:411-6. 69. Fussey SP, Bassendine MF, James OF, Yeaman SJ. Characterisation of the reactivity of autoantibodies in primary biliary cirrhosis. FEBS Lett 1989;246:49-53. 70. Kaplan MM, Rabson AR, Lee Y-M, Williams DL, Montaperto PA. Discordant occurrence of primary biliary cirrhosis in monozygotic twins. N Engl J Med 1994;331:952. 71. D’Amico E, Paroli M, Fratelli V, et al. Primary biliary cirrhosis induced by interferon-alpha therapy for hepatitis C virus infection. Dig Dis Sci 1995;40:2113-6. 72. Moradpour D, Altorfer J, Flury R, et al. Chlorpromazine-induced vanishing bile duct syndrome leading to biliary cirrhosis. Hepatology 1994; 20:1437-41. 73. Tsuneyama K, Van de Water J, Leung PS, et al. Abnormal expression of the E2 component of the pyruvate dehydrogenase complex on the luminal surface of biliary epithelium occurs before major histocompatibility complex class II and BB1/B7 expression. Hepatology 1995;21:1031-7. 74. Kneppelhout JC, Mulder CJ, van Berge Henegouwen GP, de Vries RA, Brandt KH. Ursodeoxycholic acid treatment in primary biliary cirrhosis with the emphasis on late stage disease. Neth J Med 1992;41:11-6. 75. Heuman DM, Pandak WM, Hylemon PB, Vlahcevic ZR. Conjugates of ursodeoxycholate protect against cytotoxicity of more hydrophobic bile salts: in vitro studies in rat hepatocytes and human erythrocytes. Hepatology 1991;14:920-6. 76. Sherlock S, Scheuer PJ. The presentation and diagnosis of 100 patients with primary biliary cirrhosis. N Engl J Med 1973;289:674-8. 77. Long RG, Scheuer PJ, Sherlock S. Presentation and course of asymptomatic primary biliary cirrhosis. Gastroenterology 1977;72:1204-7. 78. Balasubramaniam K, Grambsch PM, Wiesner RH, Lindor KD, Dickson ER. Diminished survival in asymptomatic primary biliary cirrhosis: a prospective study. Gastroenterology 1990;98:1567-71. 79. Tornay AS Jr. Primary biliary cirrhosis: natural history. Am J Gastroenterol 1980;73:223-6. 80. Ghent CN, Bloomer JR, Klatskin G. Elevations in skin tissue levels of bile acids in human cholestasis: relation to serum levels and to pruritus. Gastroenterology 1977;73:1125-30. 81. Bergasa NV, Talbot TL, Alling DW, et al. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology 1992;102:544-9. 82. Laurin JM, DeSotel CK, Jorgensen RA, Dickson ER, Lindor KD. The natural history of abdominal pain associated with primary biliary cirrhosis. Am J Gastroenterol 1994;89:1840-3. 83. Christensen E, Crowe J, Doniach D, et al. Clinical pattern and course of disease in primary biliary cirrhosis based on an analysis of 236 patients. Gastroenterology 1980;78:236-46. 84. Fleming CR, Dickson ER, Hollenhorst RW, Goldstein NP, McCall JT, Baggenstoss AH. Pigmented corneal rings in a patient with primary biliary cirrhosis. Gastroenterology 1975;69:220-5. 85. Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology 1989;10:1-7. 86. Ahrens EJ Jr, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis. Medicine (Baltimore) 1950;29:299-364. 87. Iannuzzi C, Cozzolino G, Negro G. Elective cholecystectomy in selected cirrhotic patients. Acta Chir Belg 1993;93:147-50. 88. Jahn CE, Schaefer EJ, Taam LA, et al. Lipoprotein abnormalities in primary biliary cirrhosis: association with hepatic lipase inhibition as well as altered cholesterol esterification. Gastroenterology 1985;89:1266-78. 89. Crippin JS, Lindor KD, Jorgensen R, et al. Hypercholesterolemia and atherosclerosis in primary biliary cirrhosis: what is the risk? Hepatology 1992;15:858-62. 90. Gregory WL, Game FL, Farrer M, Idle JR, Laker MF, James OF. Reduced serum lipoprotein(a) levels in patients with primary biliary cirrhosis. Atherosclerosis 1994;105:43-50. 91. Simon JB, Poon RW. Lipoprotein-X levels in extrahepatic versus intrahepatic cholestasis. Gastroenterology 1978;75:177-80. 92. Poupon RE, Ouguerram K, Chretien Y, et al. Cholesterol-lowering effect of ursodeoxycholic acid in patients with primary biliary cirrhosis. Hepatology 1993;17:577-82. 93. Poupon RE, Balkau B, Guechot J, Heintzmann F. Predictive factors in ursodeoxycholic acid-treated patients with primary biliary cirrhosis: role of serum markers of connective tissue. Hepatology 1994;19:635-40. 94. Olomu AB, Vickers CR, Waring RH, et al. High incidence of poor sulfoxidation in patients with primary biliary cirrhosis. N Engl J Med 1988; 318:1089-92. 95. Davies MH, Ngong JM, Pean A, Vickers CR, Waring RH, Elias E. Sulphoxidation and sulphation capacity in patients with primary biliary cirrhosis. J Hepatol 1995;22:551-60. 96. Ros E, Garcia-Puges A, Reixach M, Cuso E, Rodes J. Fat digestion and exocrine pancreatic function in primary biliary cirrhosis. Gastroenterology 1984;87:180-7. 97. Lanspa SJ, Chan AT, Bell JS III, Go VL, Dickson ER, DiMagno EP. Pathogenesis of steatorrhea in primary biliary cirrhosis. Hepatology 1985; 5:837-42. 98. Reynolds TB, Denison EK, Frankl HD, Lieberman FL, Peters RL. Primary biliary cirrhosis with scleroderma, Raynaud’s phenomenon and telangiectasia: new syndrome. Am J Med 1971;50:302-12. 99. Tsianos EV, Hoofnagle JH, Fox PC, et al. Sjogren’s syndrome in patients with primary biliary cirrhosis. Hepatology 1990;11:730-4. Vol ume 335 Numbe r 21 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved. 1579 The New England Journal of Medicine 100. Giovannini A, Ballardini G, Amatetti S, Bonazzoli P, Bianchi FB. Patterns of lacrimal dysfunction in primary biliary cirrhosis. Br J Ophthalmol 1985;69:832-5. 101. Schussler GC, Schaffner F, Korn F. Increased serum thyroid hormone binding and decreased free hormone in chronic active liver disease. N Engl J Med 1978;299:510-5. 102. Pares A, Rimola A, Bruguera M, Mas E, Rodes J. Renal tubular acidosis in primary biliary cirrhosis. Gastroenterology 1981;80:681-6. 103. Loof L, Adami HO, Sparen P, et al. Cancer risk in primary biliary cirrhosis: a population-based study from Sweden. Hepatology 1994;20: 101-4. 104. Witt-Sullivan H, Heathcote J, Cauch K, et al. The demography of primary biliary cirrhosis in Ontario, Canada. Hepatology 1990;12:98-105. 105. Butler P, Hamilton-Miller JM, McIntyre N, Burroughs AK. Natural history of bacteriuria in women with primary biliary cirrhosis and the effect of antimicrobial therapy in symptomatic and asymptomatic groups. Gut 1995;36:931-4. 106. Matloff DS, Kaplan MM, Neer RM, Goldberg MJ, Bitman W, Wolfe HJ. Osteoporosis in primary biliary cirrhosis: effects of 25-hydroxyvitamin D3 treatment. Gastroenterology 1982;83:97-102. 107. Kaplan MM, Goldberg MJ, Matloff DS, Neer RM, Goodman DB. Effect of 25-hydroxyvitamin D3 on vitamin D metabolites in primary biliary cirrhosis. Gastroenterology 1981;81:681-5. 108. Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest 1995;95:2581-6. 109. Herlong HF, Recker RR, Maddrey WC. Bone disease in primary biliary cirrhosis: histologic features and response to 25-hydroxyvitamin D. Gastroenterology 1982;83:103-8. 110. Lindor KD, Janes CH, Crippin JS, Jorgensen RA, Dickson ER. Bone disease in primary biliary cirrhosis: does ursodeoxycholic acid make a difference? Hepatology 1995;21:389-92. 111. Camisasca M, Crosignani A, Battezzati PM, et al. Parenteral calcitonin for metabolic bone disease associated with primary biliary cirrhosis. Hepatology 1994;20:633-7. 112. Guanabens N, Pares A, del Rio L, et al. Sodium fluoride prevents bone loss in primary biliary cirrhosis. J Hepatol 1992;15:345-9. 113. Kaplan MM, Elta GH, Furie B, Sadowski JA, Russell RM. Fat-soluble vitamin nutriture in primary biliary cirrhosis. Gastroenterology 1988;95: 787-92. 114. Mahl TC, Shockcor W, Boyer JL. Primary biliary cirrhosis: survival of a large cohort of symptomatic and asymptomatic patients followed for 24 years. J Hepatol 1994;20:707-13. 115. Dickson ER, Fleming TR, Wiesner RH, et al. Trial of penicillamine in advanced primary biliary cirrhosis. N Engl J Med 1985;312:1011-5. 116. Goodman ZD, McNally PR, Davis DR, Ishak KG. Autoimmune cholangitis: a variant of primary biliary cirrhosis: clinicopathologic and serologic correlations in 200 cases. Dig Dis Sci 1995;40:1232-42. 117. Beswick DR, Klatskin G, Boyer JL. Asymptomatic primary biliary cirrhosis: a progress report on long term follow-up and natural history. Gastroenterology 1985;89:267-71. 118. Christensen E, Neuberger J, Crowe J, et al. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis: final results of an international trial. Gastroenterology 1985;89:1084-91. 119. Roll J, Boyer JL, Barry D, Klatskin G. The prognostic importance of clinical and histologic features in asymptomatic and symptomatic primary biliary cirrhosis. N Engl J Med 1983;308:1-7. 120. Bachs L, Pares A, Elena M, Piera C, Rodes J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology 1992;102:2077-80. 121. Poupon RE, Poupon R, Balkau B, UDCA–PBC Study Group. Ursodiol for the long-term treatment of primary biliary cirrhosis. N Engl J Med 1994;330:1342-7. 122. Cohen LB, Ambinder EP, Wolke AM, Field SP, Schaffner F. Role of plasmapheresis in primary biliary cirrhosis. Gut 1985;26:291-4. 123. Thornton JR, Triger DR, Losowsky MS. Variceal bleeding is associated with reduced risk of severe cholestasis in primary biliary cirrhosis. Q J Med 1989;71:467-71. 124. Navasa M, Pares A, Bruguera M, Caballeria J, Bosch J, Rodes J. Portal hypertension in primary biliary cirrhosis: relationship with histological features. J Hepatol 1987;5:292-8. 125. Boyer TD, Kokenes DD, Hertzler G, Kutner MH, Henderson JM. Effect of distal splenorenal shunt on survival of patients with primary biliary cirrhosis. Hepatology 1994;20:1482-6. 126. Grambsch PM, Dickson ER, Kaplan M, LeSage G, Fleming TR, Langworthy AL. Extramural cross-validation of the Mayo primary biliary cirrhosis survival model establishes its generalizability. Hepatology 1989; 10:846-50. 127. Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, 1580 James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis: three-year results. J Hepatol 1992;15:336-44. 128. Lindor KD, Dickson ER, Baldus WP, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology 1994;106:1284-90. 129. Heathcote EJ, Cauch-Dudek K, Walker V, et al. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1994;19:1149-56. 130. Combes B, Carithers RL Jr, Maddrey WC, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1995;22:759-66. 131. Heathcote EJ, Lindor KD, Poupon R, et al. Combined analysis of French, American and Canadian randomized controlled trials of ursodeoxycholic acid therapy in primary biliary cirrhosis. Gastroenterology 1995; 108:Suppl:A1082. abstract. 132. Jorgensen RA, Dickson ER, Hofmann AF, Rossi SS, Lindor KD. Characterisation of patients with a complete biochemical response to ursodeoxycholic acid. Gut 1995;36:935-8. 133. Wiesner RH, Ludwig J, Lindor KD, et al. A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. N Engl J Med 1990; 322:1419-24. 134. Lombard M, Portmann B, Neuberger J, et al. Cyclosporin A treatment in primary biliary cirrhosis: results of a long-term placebo controlled trial. Gastroenterology 1993;104:519-26. 135. Kaplan MM, Alling DW, Zimmerman HJ, et al. A prospective trial of colchicine for primary biliary cirrhosis. N Engl J Med 1986;315:1448-54. 136. Bodenheimer HC Jr, Schaffner F, Sternlieb I, Klion FM, Vernace S, Pezzullo J. A prospective clinical trial of D-penicillamine in the treatment of primary biliary cirrhosis. Hepatology 1985;5:1139-42. 137. Warnes TW, Smith A, Lee FI, Haboubi NY, Johnson PJ, Hunt L. A controlled trial of colchicine in primary biliary cirrhosis: trial design and preliminary report. J Hepatol 1987;5:1-7. 138. Zifroni A, Schaffner F. Long-term follow-up of patients with primary biliary cirrhosis on colchicine therapy. Hepatology 1991;14:990-3. 139. Vuoristo M, Farkkila M, Karvonen AL, et al. A placebo-controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid. Gastroenterology 1995;108:1470-8. 140. Shibata J, Fujiyama S, Honda Y, Sato T. Combination therapy with ursodeoxycholic acid and colchicine for primary biliary cirrhosis. J Gastroenterol Hepatol 1992;7:277-82. 141. Kaplan MM, Knox TA. Treatment of primary biliary cirrhosis with low-dose weekly methotrexate. Gastroenterology 1991;101:1332-8. 142. Kaplan MM. Sustained remission of primary biliary cirrhosis (PBC) with methotrexate (MTX). Gastroenterology 1995;108:Suppl:A1094. abstract. 143. Kaplan M, Schmid C, McKusick A, Provenzale D, Sharma A, Sepe T. Double-blind trial of methotrexate (MTX) versus colchicine (COLCH) in primary biliary cirrhosis (PBC). Hepatology 1993;18:Suppl:176A. abstract. 144. Kaplan MM, Dickstein G, Schmid C. Methotrexate (MTX) improves histology in primary biliary cirrhosis (PBC). Hepatology 1994;20:Suppl: 152A. abstract. 145. Miller LC, Sharma A, McKusick AF, Tassoni JP, Dinarello CA, Kaplan MM. Synthesis of interleukin-1 beta in primary biliary cirrhosis: relationship to treatment with methotrexate or colchicine and disease progression. Hepatology 1995;22:518-24. 146. Kaplan MM. The therapeutic effects of ursodiol and methotrexate are additive and well tolerated in primary biliary cirrhosis (PBC). Hepatology 1992;16:Suppl:92A. abstract. 147. Buscher HP, Zietzschmann Y, Gerok W. Positive responses to methotrexate and ursodeoxycholic acid in patients with primary biliary cirrhosis responding insufficiently to ursodeoxycholic acid alone. J Hepatol 1993; 18:9-14. 148. Lindor KD, Dickson ER, Jorgensen RA, et al. The combination of ursodeoxycholic acid and methotrexate for patients with primary biliary cirrhosis: the results of a pilot study. Hepatology 1995;22:1158-62. 149. Sharma A, Provenzale D, McKusick A, Kaplan MM. Interstitial pneumonitis after low-dose methotrexate therapy in primary biliary cirrhosis. Gastroenterology 1994;107:266-70. 150. Markus BH, Dickson ER, Grambsch PM, et al. Efficacy of liver transplantation in patients with primary biliary cirrhosis. N Engl J Med 1989; 320:1709-13. 151. Gouw AS, Haagsma EB, Manns M, Klompmaker IJ, Slooff MJ, Gerber MA. Is there recurrence of primary biliary cirrhosis after liver transplantation? A clinicopathologic study in long-term survivors. J Hepatol 1994; 20:500-7. 152. Balan V, Batts KP, Porayko MK, Krom RA, Ludwig J, Wiesner RH. Histological evidence for recurrence of primary biliary cirrhosis after liver transplantation. Hepatology 1993;18:1392-8. 153. Kaplan MM. Primary biliary cirrhosis — a first step in prolonging survival. N Engl J Med 1994;330:1386-7. Novem b er 2 1 , 1 9 9 6 Downloaded from www.nejm.org at LIBRARIES OF THE UNIV OF COLORADO on May 4, 2010 . Copyright © 1996 Massachusetts Medical Society. All rights reserved.