* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A human immunodeficiency virus type 1 Env–granulocyte
Survey
Document related concepts
Drosophila melanogaster wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Immunocontraception wikipedia , lookup
Immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Molecular mimicry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Transcript
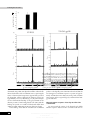
Journal of General Virology (1999), 80, 217–223. Printed in Great Britain ................................................................................................................................................................................................................................................................................... A human immunodeficiency virus type 1 Env–granulocytemacrophage colony-stimulating factor fusion protein enhances the cellular immune response to Env in a vaccinia virus-based vaccine Dolores Rodrı! guez,1 Juan Ramo! n Rodrı! guez,1 Mercedes Llorente,2 Isabel Va! zquez,1 Pilar Lucas,2 Mariano Esteban,1 Carlos Martı! nez-A.2 and Gustavo del Real2 Departments of Molecular & Cellular Biology1 and Immunology & Oncology2, Centro Nacional de Biotecnologı! a, CSIC/UAM, Campus de Cantoblanco, E-28049 Madrid, Spain Vaccinia virus (VV) infection induces protective T- and B-cell responses, making recombinants based on VV good candidates for the development of effective vaccines to other viruses. VV recombinants expressing the human immunodeficiency virus (HIV) envelope protein (Env) have been generated in several laboratories and shown to induce anti-HIV cellular and humoral immune responses in vaccinated humans and in chimpanzees. To increase the immunogenicity of the Env antigen, a VV recombinant was generated that expresses a chimeric antigen consisting of the Env protein fused to an immunostimulatory cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF). The chimeric protein retained GM-CSF biological activity when expressed by this recombinant virus (VV-GM–gp120) in cells infected in vitro. Infection of BALB/c mice with VVGM–gp120 triggered a higher HIV-specific cellular immune response, as measured by interferonγ production, than that induced by a VV recombinant expressing the native Env protein. Moreover, although anti-gp120 antibody titres were similar in sera from mice inoculated with either of the VV recombinants, immunization with the recombinant expressing the fusion protein elicited antibodies against a broader spectrum of Env epitopes. These results indicate that HIV Env antigen fusion to GM-CSF provides a means to improve the anti-HIV immune response. Introduction Vaccinia virus (VV), the best-characterized member of the family Poxviridae, has proven to be an effective vaccine, as its use has resulted in the global eradication of smallpox (Fenner et al., 1988). The possibility of adverse effects after vaccination with VV has been minimized by inactivation of genes encoding virulence factors (Moss, 1996 ; Paoletti, 1996). VV recombinants expressing human immunodeficiency virus (HIV) antigens have been developed in several laboratories (Chakrabarty et al., 1986 ; Hu et al., 1986 ; Rodrı! guez et al., 1989) in order to investigate their potential utility as vaccines against HIV infection. Phase I clinical trials in healthy individuals vaccinated with different HIV-1 envelope protein (Env) poxvirus recombinants demonstrated that such a vaccine is safe and induces Author for correspondence : Mariano Esteban. Fax j34 91 585 4506. e-mail mesteban!cnb.uam.es 0001-5728 # 1999 SGM humoral and T-cell responses specific for this HIV antigen (Cooney et al., 1993 ; Graham et al., 1993 ; McElrath et al., 1994 ; Pioloux et al., 1995 ; Egan et al., 1995 ; Fleury et al., 1996). Most of these reports describe an enhancement of the anti-Env immune response when individuals primed with a poxvirus recombinant were boosted with purified recombinant gp160. These experiments suggest that, although such a strategy appears promising, a more effective primary response would be of considerable importance. In an attempt to improve presentation of HIV antigen to the host immune system, we have generated a VV recombinant expressing a chimeric HIV Env–granulocyte-macrophage colony-stimulating factor (GMCSF) fusion protein. GM-CSF is a haematopoietic factor that induces proliferation of haematopoietic precursor cells and their differentiation to neutrophils, eosinophils and macrophages (Gough et al., 1984 ; Metcalf, 1986). It is also the main stimulatory Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Mon, 19 Jun 2017 01:17:08 CBH D. Rodrı! guez and others cytokine for the viability, differentiation and function of Langerhans’ and dendritic cells (Sallusto & Lanzavecchia, 1994), which are the most important cell types for the stimulation in vivo of primary T-cell-mediated immune responses (Steinman, 1991). GM-CSF also enhances monocyte surface MHC class II expression, adhesion molecules and co-stimulatory factors, and stimulates interleukin-1 secretion, critical for antigen presentation to T lymphocytes (Smith et al., 1990). The immunostimulatory activity of GM-CSF permits enhancement of T-cell proliferative responses to suboptimal antigen concentrations and an increase in primary antibody responses (Morrissey et al., 1987 ; Grabstein et al., 1996 ; Disis et al., 1996 ; Kim et al., 1997). GM-CSF has also been shown to increase tumour immunogenicity in animal models (Dranoff et al., 1993). Cytokinetransduced autologous melanoma cells have been used as a therapeutic vaccine in humans, resulting in the generation of an anti-tumour immune response associated with clinical benefit (Ellem et al., 1997). Here we report that immunization with a VV recombinant expressing GM-CSF fused to HIV-1 Env resulted in an augmented cellular immune response compared with that elicited by a VV recombinant expressing the Env protein alone. Both HIV-Env-specific T-cell proliferation and the number of interferon-γ (IFN-γ)-producing CD8+ cells increased in mice immunized with the fusion protein, indicating that T-cellmediated immune responses were augmented by the presence of GM-CSF. Although serum antibody titres were similar in mice infected with either of the VV recombinants, immunization with the fusion protein elicited antibodies against a broader spectrum of gp120 epitopes. The combined effect of the increase in these responses and the more extensive antibody repertoire triggered by GM-CSF may be critical in promoting a neutralizing response to HIV-1. Methods Cells and viruses. African green monkey kidney cells (BSC-40) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % newborn calf serum. P815 mastocytoma cells, which express MHC class I molecules, were cultured in DMEM containing 10 % foetal calf serum (FCS). The VV strain WR was propagated and titrated in BSC-40 cells. The recombinant virus VV-ENV, expressing the entire env gene of HIV-1 (strain IIIB), has been described previously (Rodrı! guez et al., 1989). The human lymphoid cell line MT2 was grown in RPMI supplemented with 10 % FCS. VV recombinant construction. Generation of the VV insertion vectors VV-GM-CSF and VV-GM–gp120 is explained in the legend to Fig. 1. The GM-CSF and GM–gp120 genes were inserted into the thymidine kinase (TK) region of the VV WR strain by DNA homologous recombination. β-Galactosidase-producing plaques were picked, cloned three times and amplified in BSC-40 cells in culture. Recombinant viruses were purified by banding in sucrose gradients, as previously described (Rodrı! guez et al., 1989). Determination of GM-CSF biological activity. The biological activity of the GM–gp120 fusion protein was assayed by testing its ability to support proliferation of the murine GM-CSF-dependent cell line CBI NFS-60. Cells (10&\ml) were seeded into 96-well plates with dilutions of supernatants from cell cultures infected with VV-GM–gp120 or infected with a VV recombinant (VV-GM-CSF) expressing native GM-CSF. After 24 h incubation, 10 ml MTT solution (5 mg\ml 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide in PBS ; Sigma) was added and the plates were incubated for 4 h at 37 mC. The reaction was terminated by addition of 100 ml SDS\HCl solution (10 % SDS in 10 mM HCl) and the plates were incubated overnight at 37 mC. Absorbance was measured at 550 nm with a reference wavelength of 690 nm. Immunization. Six- to eight-week-old female BALB\c mice, bred in the CNB animal facility, were used. Groups of mice (three per group) were primed by infection with 5i10( p.f.u. VV inoculated by the intraperitoneal route. Animals were boosted intraperitoneally 21 days later with HIV-1 IIIB-derived soluble gp120 (5 µg per animal ; Intracel). Synthetic peptides. The peptide GPGRAFVTI, an H-2d-restricted CD8+ T-cell epitope corresponding to the V3 loop of HIV-1 strain IIIB, was generously provided by Margarita del Val (Inst. Salud Carlos III, Madrid). A collection of 47 overlapping peptides (20-mers with a 10amino-acid overlap) covering the entire gp120 protein was provided by the AIDS Reagent Project (Medical Research Council, UK ; repository reference ADP740\1-47). ELISA determination of anti-gp120 antibodies in mouse sera. ELISA microtitre plates (Maxi-sorb, Nunc) were coated by overnight incubation at 4 mC with purified gp120 (1 µg\ml ; strain IIIB, Intracel) or peptides (10 µg\ml) in PBS. After blocking with 0n5 % BSA in PBS, wells were washed with distilled water and incubated for 60 min at 37 mC with diluted mouse sera. Antibody reactivity was determined after incubation with peroxidase-labelled goat anti-mouse IgG and addition of O-phenylenediamine dihydrochloride (Sigma). The reaction was terminated with 3 M sulphuric acid and absorbance was determined at 492 nm. The titre was expressed as the highest serum dilution giving an absorbance value three times that of the preimmune serum. Cell proliferation assay. Spleens were removed from infected mice and single-cell suspensions were prepared in complete medium (RPMI 1640 with 10 % FCS, 2 mM -glutamine and 10 µM 2mercaptoethanol). Splenocytes (10' per well) were cultured in 96-well microtitre plates. Cultures were challenged in triplicate with 2 µg\ml gp120 and incubated for 3 days at 37 mC with 5 % CO , after which 1 µCi # [$H]thymidine (5 Ci\mmol ; Amersham) was added to each well. After 16 h, cells were harvested and [$H]thymidine incorporation into DNA was measured by liquid-scintillation counting. Concanavalin A (1 mg\ml ; Sigma) was used as a polyclonal stimulator control. The stimulation index (SI) was determined as the ratio (experimental count – spontaneous count)\spontaneous count. ELISPOT assay. The ELISPOT assay was used to detect epitopespecific IFN-γ-secreting cells, as previously described (Miyahira et al., 1995). Briefly, 96-well nitrocellulose-bottomed plates were coated with anti-mouse IFN-γ MAb R4-6A2 (8 µg\ml, Pharmingen). After overnight incubation at room temperature, wells were washed three times with RPMI 1640 and 100 µl medium supplemented with 10 % FCS was added to each well. Plates were then incubated at 37 mC for 1 h. Duplicate cultures were prepared from immunized mouse splenocytes in serial doubling dilutions, beginning with 10' cells per well. P815 (H-2d) cells were used as antigen-presenting cells (APC) and were pulsed with 10−' M synthetic peptide GPGRAFVTI and treated with mitomycin C (30 µg\ml, Sigma). After several washes with culture medium, 10& P815 cells were added to each well. P815 cells not pulsed with the peptide but cultured under similar conditions were used as a control. Plates were incubated for 26–28 h at 37 mC and then washed with PBS containing 0n05 % Tween 20 (PBS-T) before being incubated overnight at 4 mC with Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Mon, 19 Jun 2017 01:17:08 Immunostimulation by HIV-1 Env–GM-CSF Fig. 1. Scheme for the construction of VV insertion vectors containing the murine GM-CSF coding sequence or the GM–gp120 chimeric gene. A DNA fragment containing the GM-CSF gene was excised from plasmid pBacPAKGM-CSF by restriction with XhoI and EcoRI. This fragment was either cloned directly into the SmaI site of the VV insertion vector pSC11, generating pSCGM-CSF, or digested with SpeI to eliminate the GM-CSF stop codon (denoted by an asterisk) and cloned into plasmid pBacPAK-gp120, which had been previously digested with XhoI and SpeI to eliminate the gp120 signal peptide (SP). The chimeric gene was excised by digestion of the resulting plasmid, pBacPAKGM–gp120, with XhoI and EcoRI, treated with Klenow and cloned into pSC11 to generate the pSC-GM–gp120 VV transfer vector. The GM-CSF gene and the GM–gp120 chimeric sequence were introduced into the thymidine kinase (TK) locus of the VV genome after transfection of VV-infected cells with the plasmids pSC-GM-CSF and pSC-GM–gp120, respectively. (b) 5 1.0 gp160 gp120 0.8 0.6 0.4 Results 0.2 Analysis of protein expression by recombinant VV 0.0 The strategy for generation of VV recombinants expressing GM-CSF and the GM–gp120 fusion protein is outlined in Fig. 1. To demonstrate that VV-GM–gp120 expressed the fusion protein, BSC-40 cells were infected with VV-GM-CSF, VVGM–gp120 or VV-ENV, cell extracts were separated by SDS–PAGE and Western blots were probed with a polyclonal anti-gp120 antibody (ADP 422, MRC AIDS Reagent Project). In VV-ENV-infected cells, the anti-gp120 antibody reacted with the Env precursor gp160 and the gp120 processed form (Fig. 2 a, lane 5) but in cells infected with different VVGM–gp120 isolates (lanes 2–4), the antibody reacted with a protein with an apparent molecular mass of 150 kDa. In an ELISA in which the GM-CSF portion of the fusion protein was captured by a rat anti-GM-CSF antibody (Endogen), the gp120 moiety was revealed with two different monoclonal antibodies (F7, an anti-V2 produced in our laboratory and NEA-9284, an anti-V3 from Dupont) and two rabbit polyclonal anti-gp120 antisera (ADP 422 and CNB.1, produced in our laboratory), VV-GM-CSF VV-GM– gp120 VV-ENV 1/100 1/200 1/400 1/800 1/1600 1/3200 1/6400 1/12800 1/25600 1/51200 (a) 1 2 3 4 A550 biotinylated anti-mouse IFN-γ MAb XMG1.2 (2 µg\ml, Pharmingen) in PBS-T. Plates were then washed with PBS-T and 100 µl 1\800-diluted peroxidase-labelled avidin (Sigma) in PBS-T was added to each well. Wells were washed 1 h later with PBS-T and PBS. The spots were developed by adding 50 mM Tris–HCl (pH 7n5) containing 1 µg\ml 3,3h-diaminobenzidine tetrahydrochloride (Sigma) and 0n015 % H O . # # When the plates were completely dry, the number of spots was determined with the aid of a stereo-microscope. Supernatant dilution Fig. 2. Expression of biologically active GM–gp120 fusion protein in VVGM–gp120-infected cells. (a) Western blot analysis of BSC-40 cells infected (5 p.f.u. per cell) with VV-GM–gp120 or a recombinant virus, VVENV, expressing the entire HIV-1 Env protein. Cell extracts prepared at 24 h post-infection were fractionated on 10 % SDS–PAGE gels, transferred to nitrocellulose and incubated with gp120-specific rabbit polyclonal antibodies. Lane 1, extract from cells infected with wild-type VV ; 2–4, extracts from cells infected with VV-GM–gp120 isolates ; 5, extract from cells infected with VV-ENV. (b) Biological activity of GM-CSF and the GM–gp120 recombinant protein. Supernatants from BSC-40 cells infected for 24 h with VV-GM-CSF or VV-GM–gp120 recombinants were tested as a source of GM-CSF, which allows proliferation of the murine GM-CSFdependent NFS-60 cell line. Proliferation was quantified as described in Methods. indicating that the antigen was properly expressed (data not shown). The biological activity of GM-CSF and the chimeric GM–gp120 protein expressed by the VV recombinants was Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Mon, 19 Jun 2017 01:17:08 CBJ Anti-gp120 IgG titre (× 10 – 4) D. Rodrı! guez and others 4 (a) 3.5 3 2.5 2 1.5 1 0.5 0 (b) VV-GM-CSF VV-GM–gp120 VV-ENV VV-GM –gp120 VV-ENV 2.5 1.6 1.4 2 1.2 A492 A492 1 0.8 0.6 0.4 0.2 0 2.5 1.5 1 0.5 1.2 1 2 A492 A492 0.8 1.5 0.4 0.5 0.2 2.5 1.6 1.4 2 1.2 1.5 1 Fig. 3. Humoral 0.8 anti-HIV-1 gp120 immune response elicited in mice immunized with VV-Env recombinants. Groups of mice (three per group) were primed 0.6 by vaccination (5i107 p.f.u.) with recombinants VV-ENV, VV-GM–gp120 0.4or VV-GM-CSF. Mice were boosted 21 days later with soluble gp120 (5 µg per mouse) and serum samples were collected 29 days later. (a)0.2Anti-gp120 reactivity in pooled serum samples was analysed by ELISA with recombinant gp120-coated plates, as described in Methods. (b) Epitope mapping of mouse sera. Sera from three individual mice immunized with VV-ENV or VV-GM–gp120 were tested by ELISA for reactivity against a series of overlapping synthetic peptides gp 120 overlapping peptides covering the entire HIV Env protein. A492 A492 1 1 0.5 gp 120 overlapping peptides determined using a GM-CSF-dependent cell line. Cultured cells infected with either of the recombinant viruses, expressing the native or chimeric GM-CSF proteins, supported the growth of the dependent cell line, although the chimeric protein was approximately 50 % less efficient (Fig. 2 b). By FACS analysis we have demonstrated binding of the gp120 domain of the chimeric protein to CD4-bearing human cells (MT2) and this binding was specific, as it could be blocked with soluble CD4 (ADP 609, MRC AIDS Reagent Project) (data not shown). These findings show that the VV-GM-CSF recombinant CCA 0.6 produces a biologically active GM-CSF molecule and that the VV-GM–gp120 recombinant expresses a protein of approximately 150 kDa that exerts GM-CSF activity, binds to human CD4 receptor and is recognized by poly- and monoclonal antigp120 antibodies. Humoral immune response elicited by the HIV-1 Env recombinants We next tested the extent of the humoral and cellular immune responses induced in mice vaccinated with the VV Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Mon, 19 Jun 2017 01:17:08 Immunostimulation by HIV-1 Env–GM-CSF Chimeric GM–Env enhances the specific cellular immune response to gp120 T-helper-cell activation and proliferation is essential in inducing both humoral immune responses via B cells and cellular immune responses via CD8+ cytotoxic T cells. It was thus of interest to determine the extent of the HIV-1-specific Tcell-mediated immune response in the vaccinated mice. Two weeks after boosting, T-cell proliferation was examined in splenocytes from VV-immunized mice. Immunization with VV expressing the chimeric protein (VV-GM–gp120) significantly increased the T-cell response to Env protein compared with that of VV-ENV-immunized mice, with SI of 13n7 and 6n1, respectively (Fig. 4a). Control mice immunized with VV expressing GM-CSF did not induce T-cell proliferative responses after stimulation with soluble gp120. ELISPOT assays were performed with splenocytes from VV recombinantimmunized mice to study further the enhancement of cellular activity. Spleen cells from mice immunized with VV-GM– gp120 showed a twofold increase in the number of IFN-γsecreting cells compared with those from mice immunized with VV-ENV (68\10& versus 36\10&) ; VV-GM-CSF-immunized mice showed only 2\10& (Fig. 4 b). These results indicate that the GM-CSF portion of the chimeric protein potentiates the VV recombinant-induced Tcell response to HIV-1 Env (Fig. 4). Discussion VV recombinants expressing HIV-1 envelope antigens were one of the first prototype vaccine constructs generated against AIDS (Chakrabarty et al., 1986 ; Hu et al., 1986). In animal models, these constructs elicit both humoral and cellular immune responses (Earl et al., 1989). Virus-specific cytotoxic T lymphocytes (CTL) are generally believed to be the best candidates for control of virus replication. The dramatic decrease in HIV-1 virus load following the initial appearance of CTL after primary infection and the temporal association between HIV-1-specific CTL activity and stable virus load or 14 Stimulation index 12 (a) 10 8 6 4 2 0 70 IFN-γ secreting cells/10 5 spleen cells recombinants. Anti-gp120 reactivity in pooled serum was analysed by ELISA in 96-well gp120-coated plates. Both VVENV and VV-GM–gp120 vaccination elicited similar levels of anti-HIV-1 Env antibodies (Fig. 3a) ; the anti-gp120 antibody titre in VV-ENV-immunized mice was 3n7i10%, while that for VV-GM–gp120 was 3n2i10%. To characterize the response further, sera from both groups were tested against a panel of overlapping peptides encompassing the entire gp120 protein. Different recognition patterns were observed ; while sera from VV-ENV-immunized mice reacted only with peptides of the third hypervariable loop (V3) (peptides 27–28 corresponding to amino acids 262–290), antibodies induced by the VV-GM–gp120 immunogen recognized not only the V3 loop but also other regions, specifically the gp120 C-terminal domain (peptides 46 and 47, amino acids 452–479) (Fig. 3 b). 60 VV-GM-CSF VV-GM – gp120 VV-ENV VV-GM – gp120 VV-ENV (b) 50 40 30 20 10 0 VV-GM-CSF Fig. 4. Cellular anti-HIV-1 gp120 immune response induced after vaccination of mice with VV-Env recombinants. (a) Proliferative response of spleen cells from mice primed and boosted with VV recombinants as indicated in the legend to Fig. 3. Spleens were removed 14 days after boosting and spleen cells were incubated with (filled bars) or without (hatched bars) purified gp120 (1 µg/ml). [3H]Thymidine incorporation was measured and the SI was calculated as described in Methods. (b) V3 loop-specific CD8+ T-cell response in spleen cells from mice immunized as described above. The number of IFN-γ-secreting CD8+ cells was quantified by the ELISPOT assay, following the protocol described in Methods, after stimulation of the splenocytes with V3 loop peptidepresenting APC. CD4+ cell counts during asymptomatic stages are the best indicators of CTL efficiency. A good correlation has also recently been reported between long-term survival and CTL activity in paediatric HIV-1-infected patients (Luzuriaga & Sullivan, 1993). The mechanisms involved in this CTL protection include the elimination of virus-infected cells and production of suppressive factors, creating a balance between virus elimination and replication. We have studied the potential enhancement of immune responses to HIV-1 gp120 in mice immunized with a chimeric GM-CSF–gp120 protein expressed by a recombinant VV. Immunization with antigens fused to GM-CSF facilitates primary immune responses (Tao & Levy, 1993) and, by using two previously characterized HIV-1 MN vaccine constructs, Ahlers et al. (1997) showed that GM-CSF is the most effective cytokine for enhancing cellular and humoral immunity. Here, we generated VV recombinants expressing murine GM-CSF (VV-GM-CSF), the HIV-1 Env protein (VV-ENV) or a chimeric antigen consisting of the HIV-1 Env protein fused to GM-CSF (VV-GM–gp120). Western blot analysis of VV-GM–gp120infected cells showed a product of approximately 150 kDa that was recognized by different gp120-specific antibodies. The Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Mon, 19 Jun 2017 01:17:08 CCB D. Rodrı! guez and others fusion protein was efficiently released to the medium from cells and was biologically active, since culture supernatants from infected cells promoted proliferation of a GM-CSF-dependent cell line. This activity was somewhat lower, however, compared with the activity of native GM-CSF expressed by VVGM-CSF. The gp120 moiety retained its ability to bind specifically to the human CD4 receptor. BALB\c mice immunized with VV-GM–gp120 developed a broader spectrum of anti-gp120 antibodies than animals immunized with a VV recombinant expressing the non-fused Env antigen (VV-ENV). Epitopes corresponding to the V3 loop of the envelope protein were recognized equally well by serum from both groups of mice. In addition, VV-GM–gp120induced antibodies recognized peptides corresponding to the C terminus of gp120 that were not recognized by antibodies elicited after VV-ENV immunization. These results could be explained by differential folding and presentation of the HIV Env protein to the immune system when fused to GM-CSF. Although the V3 loop is the principal neutralizing linear determinant in the Env protein, the sequence variability of this segment gives rise to limited antibody specificity, reducing the potential utility of a protective antibody response (Goudsmit et al., 1991). This problem has intensified interest in neutralization sites outside the V3 loop. Neutralizing epitopes have been reported in the gp120 C terminus, in a highly immunogenic region of gp41 and in the C terminus of gp41 (Ho et al., 1987). We also observed enhancement of the anti-gp120 cellular immune response in animals immunized with VV expressing the chimeric GM–gp120 antigen. Both the T-cell proliferative responses and the number of IFN-γ-secreting cells, as determined in the ELISPOT assay, showed an approximately twofold increase in splenocytes from animals immunized with the fusion protein when compared with those of animals immunized with Env alone. The results of the ELISPOT assay specifically indicate an increase in the anti-gp120 CD8+ T-cell response induced by the recombinant virus expressing the chimeric GM–gp120 antigen. These results show that GM-CSF contributes to the enhancement of the cellular immune response against the HIV1 Env antigen elicited by recombinant VV and that coexpression of this immunomodulatory cytokine may represent a means of improving the efficiency of VV-based anti-HIV-1 vaccines. Chakrabarty, S., Robert-Guroff, M., Wong-Stall, F., Gallo, R. C. & Moss, B. (1986). Expression of the HTLV-III envelope gene by a recombinant vaccinia virus. Nature 320, 535–537. Cooney, E. L., McElrath, J. M., Corey, L., Hu, S.-L., Collier, A. C., Arditti, D., Hoffman, M., Coombs, R. W., Smith, G. E. & Greenberg, P. D. (1993). Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regime consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proceedings of the National Academy of Sciences, USA 90, 1882–1886. Disis, M. L., Bernhard, H., Shiota, F. M., Hand, S. L., Gralow, J. R., Huseby, E. S., Gillis, S. & Cheever, M. A. (1996). Granulocyte- macrophage colony-stimulating factor : an effective adjuvant for protein and peptide-based vaccines. Blood 88, 202–210. Dranoff, G., Jaffee, E., Lazenby, A., Golumbek, P., Levitsky, H., Brose, K., Jackson, V., Hamada, H., Pardoll, D. & Mulligan, R. C. (1993). Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences, USA 90, 3539–3543. Earl, P. L., Robert-Guroff, M., Matthews, T. J., Krohn, K., London, W. T. & Moss, B. (1989). Isolate- and group-specific immune responses to the envelope protein of human immunodeficiency virus induced by a live recombinant vaccinia virus in macaques. AIDS Research and Human Retroviruses 5, 23–32. Egan, M. A., Pavlat, W. A., Tartaglia, J., Paoletti, E., Weinhold, K. J., Clements, M. L. & Siliciano, R. F. (1995). Induction of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T lymphocyte responses in seronegative adults by a nonreplicating, host-rangerestricted canarypox vector (ALVAC) carrying the HIV-1MN env gene. Journal of Infectious Diseases 171, 1623–1627. Ellem, K. A., O’Rourke, M. G., Johnson, G. R., Parry, G., Misko, I. S., Schmidt, C. W., Parsons, P. G., Burrows, S. R., Cross, S., Fell, A., Li, C. L., Bell, J. R., Dubois, P. J., Moss, D. J., Good, M. F., Kelso, A., Cohen, L. K., Dranoff, G. & Mulligan, R. C. (1997). A case report : immune responses and clinical course of the first human use of granulocyte\macrophage-colony-stimulating-factor-transduced autologous melanoma cells for immunotherapy. Cancer Immunology Immunotherapy 44, 10–20. Fenner, F., Henderson, D. A., Arita, I., Jezek, Z. & Ladnyi, I. D. (1988). Smallpox and Its Eradication. Geneva : World Health Organization. Fleury, B., Janvier, G., Pialoux, G., Buseyne, F., Robertson, M. N., Tartaglia, J., Paoletti, E., Kieny, M. P., Excler, J. L. & Riviere, Y. (1996). Memory cytotoxic T lymphocyte responses in human immunodeficiency virus type 1 (HIV-1)-seronegative volunteers immunized with a recombinant canarypox expressing gp160 of HIV-1 and boosted with a recombinant gp160. Journal of Infectious Diseases 174, 734–738. Goudsmit, J., Back, N. K. T. & Nara, P. L. (1991). Genomic diversity and antigenic variation of HIV-1 : links between pathogenesis, epidemiology and vaccine development. FASEB Journal 5, 2427–2436. We are grateful to C. Mark for kind editorial assistance and V. Jimenez for technical assistance. The Department of Immunology and Oncology was founded and is supported by the CSIC and Pharmacia & Upjohn. This work was supported in part by a grant from CICYT of Spain SAF95-0072 (to M. E.). D. R. and J. R. R. were recipients of contracts from the CSIC of Spain. encoding a murine haematopoietic growth regulator, granulocytemacrophage colony stimulating factor. Nature 309, 763–767. References production in vitro by granulocyte-macrophage colony stimulating factor. Journal of Molecular and Cellular Immunology 2, 199–207. Ahlers, J. D., Dunlop, N., Alling, D. W., Nara, P. L. & Berzofsky, J. A. (1997). Cytokine-in-adjuvant steering of the immune response pheno- type to HIV-1 vaccine constructs. Journal of Immunology 158, 3947–3958. CCC Gough, N. M., Gough, J., Metcalf, D., Kelso, A., Grail, D., Nicola, N. A., Burgess, A. W. & Dunn, A. R. (1984). Molecular cloning of cDNA Grabstein, K., Mochizuki, D., Kronheim, S., Price, V., Cosman, D., Urdal, D., Gillis, S. & Conlon, P. (1986). Regulation of antibody Graham, B. S., Matthews, T. J., Belshe, R. B., Clements, M. L., Dolin, R., Wright, P. F., Gorse, G. J., Schwartz, D. H., Keefer, M. C., Bolognesi, D. P., Corey, L., Stablein, D. M., Esterliz, J. R., Hu, S.-L., Smith, G. E., Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Mon, 19 Jun 2017 01:17:08 Immunostimulation by HIV-1 Env–GM-CSF Fast, P. E. & Koff, W. C. (1993). Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. The NIAD AIDS Vaccine Clinical Trials Network. Journal of Infectious Diseases 167, 533–537. Ho, D. D., Sarngadharan, M. G., Hirsch, M. S., Schooley, R. T., Rota, T. R., Kennedy, R. C., Chanh, T. C. & Sato, V. L. (1987). Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. Journal of Virology 61, 2024–2028. Hu, S. L., Kosowski, S. G. & Dalrymple, J. M. (1986). Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature 320, 537–539. Kim, J. J., Ayyavoo, V., Bagarazzi, M. L., Chattergoon, M. A., Dang, K., Wang, B., Boyer, J. D. & Weiner, D. B. (1997). In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. Journal of Immunology 158, 816–826. Luzuriaga, K. & Sullivan, J. L. (1993). HIV-1 specific CTL in long-term survivors of vertical infection. Journal of Cellular Biochemistry Supplement 17E, 98. McElrath, M. J., Corey, L., Berger, D., Hoffman, M. C., Klucking, S., Dragavon, J., Peterson, R. & Greenberg, P. D. (1994). Immune responses elicited by recombinant vaccinia-human immunodeficiency virus (HIV) envelope and HIV envelope proteins : analysis of the durability of responses and effect of repeated boosting. Journal of Infectious Diseases 169, 41–47. Metcalf, D. (1986). The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood 67, 257–267. Miyahira, Y., Murata, K., Rodrı! guez, D., Rodrı! guez, J. R., Esteban, M., Rodrigues, M. M. & Zavala, F. (1995). Quantification of antigen specific CD8+ T cells using an ELISPOT assay. Journal of Immunological Methods 181, 45–54. mary antibody response by enhancing the function of antigen-presenting cells. Journal of Immunology 139, 1113–1119. Moss, B. (1996). Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proceedings of the National Academy of Sciences, USA 93, 11341–11348. Paoletti, E. (1996). Applications of poxvirus vectors to vaccination : an update. Proceedings of the National Academy of Sciences, USA 93, 11349–11353. Pialoux, G., Excler, J.-L., Riviere, Y., Gonzalez-Canali, G., Feuillie, V., Coulaud, P., Gluckman, J.-C., Matthews, T. J., Meignier, B., Kieny, M.P., Gonnet, P., Diaz, I., Meric, C., Paoletti, E., Tartaglia, J., Salomon, H. & Plotkin, S. (1995). A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN\LAI). AIDS Research and Human Retroviruses 11, 373–381. Rodrı! guez, D., Rodrı! guez, J. R., Rodrı! guez, J. F., Trauber, D. & Esteban, M. (1989). Highly attenuated vaccinia virus mutants for the generation of safe recombinant viruses. Proceedings of the National Academy of Sciences, USA 86, 1287–1291. Sallusto, F. & Lanzavecchia, A. (1994). Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocytemacrophage colony-stimulating factor plus interleukin-4 and downregulated by tumor necrosis factor-α. Journal of Experimental Medicine 179, 1109–1118. Smith, P. D., Lamerson, C. L., Wong, H. L., Wahl, L. M. & Wahl, S. M. (1990). Granulocyte-macrophage colony-stimulating factor stimulates human monocyte accessory cell function. Journal of Immunology 144, 3829–3834. Steinman, R. (1991). The dendritic cell system and its role in immunogenicity. Annual Review of Immunology 9, 271–296. Tao, M. H. & Levy, R. (1993). Idiotype\granulocyte-macrophage colony-stimulating factor fusion protein as a vaccine for B-cell lymphoma. Nature 362, 755–758. Morrissey, P. J., Bressler, L., Park, L. S., Alpert, A. & Gillis, S. (1987). Granulocyte-macrophage colony-stimulating factor augments the pri- Received 2 June 1998 ; Accepted 2 September 1998 Downloaded from www.microbiologyresearch.org by IP: 88.99.165.207 On: Mon, 19 Jun 2017 01:17:08 CCD