* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PATH 417 Case 3 Week 1: The Body System- Hasrit
Adaptive immune system wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Immune system wikipedia , lookup
Inflammation wikipedia , lookup
Urinary tract infection wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Tuberculosis wikipedia , lookup
Common cold wikipedia , lookup
Hepatitis B wikipedia , lookup
Neonatal infection wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Innate immune system wikipedia , lookup
Infection control wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
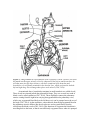
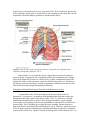
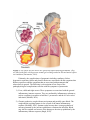
PATH 417 Case 3 Week 1: The Body System- Hasrit Sidhu Overview of the Case Based on the signs and symptoms presented, it is likely that Robert will be diagnosed with pneumonia or tuberculosis. Pneumonia is an infection of the lungs, which results in the inflammation of the air sacs (MayoClinic, 2015). In this condition, the air sacs are filled with fluid or pus and, as a result, the patient experiences cough with excessive sputum production, fever, chills and difficulty in breathing (MayoClinc, 2015). Pneumonia can be divided into two forms: 1) Bronchial Pneumonia: This involves the alveoli contiguous to the larger bronchioles of the bronchial tree and occurs in infants, young children and aged adults. 2) Lobar Pneumonia: This involves only a single lobe of the lung and is prevalent in young adults (Todar, 2012). Pneumonia can be caused by a variety of organisms, including bacteria, viruses and fungi (MayoClinic, 2015). For the purposes of this course and Robert’s case, we will focus on bacterial causes. The primary bacterial cause of pneumonia is Streptococcus pneumonia (Figure 1), which forms a component of the normal flora in the nose and throat (Healthline, 2015). When the immune system is weakened or the respiratory system is damaged, possibly by the inflammatory immune response, the bacteria are able to enter the lungs by inhalation (Healthline, 2015). It is important to note that there are other bacterial causes of pneumonia; however, for the purposes of this report, we will limit our discussion to S. pneumoniae, which is the most common cause of pneumonia. These signs and symptoms can also be associated with tuberculosis. Tuberculosis is an infectious disease that affects the lungs and is usually caused by the bacterium, Mycobacterium tuberculosis (Figure 1) (Tuberculosis, 2016). Throughout this report, we will discuss the signs and symptoms, the affect on normal physiological functioning and the secondary infections associated with both of these bacteria. Figure 1: A scanning electron micrograph of S. pneumoniae (left) and M. tuberculosis (right) (Todar, 2012). Signs and Symptoms A sign is an objective characteristic that is identified by a healthcare professional, such as a physician (Niamh and Lowe, 2016). Patients may or may not be aware of the signs that are associated with their illness (Niamh and Lowe, 2016). In contrast, a symptom is a characteristic that the patient observes or feels (Niamh and Lowe, 2016). Based on this, there are some characteristics of the disease, which are detected, by the physician and not the patient and there are some characteristics that are detected by patient and not detected by the physician. Finally, there are also some disease characteristics that are both signs and symptoms. Please consult the following table for Robert’s signs and symptoms: - - - - Signs A fever of 38.5 degrees Celsius The presence of crackles in the right lung. These crackles usually reflect a build up of fluid in the lungs. Fluid buildup may be due to the congregation of white blood cells in response to the infection in the lung (LiveStrong, 2015) There are decreased breath sounds in the right lower lung field. Breath sounds are the noises produced by the structures of the lungs during breathing. Decreased breath sounds can mean that there is fluid in the lung as a result of the immune response to bacterial infection (MedlinePlus, 2015). Lab identification of the pathogen via X-Rays and the deep sputum tests. A chronic productive cough, which may result in the coughing up of mucous/sputum from the lung. The mucous may be green or it may contain some blood (WebMD, 2014). Increased heart rate (WebMD, 2014). Symptoms - - A fever Chills Night Sweats A chronic productive cough, which may result in the coughing up of mucous/sputum from the lung. The mucous may be green or it may contain some blood (WebMD, 2014). Shortness of breath (WebMD, 2014). Chest pain that worsens with coughing and breathing (WebMD, 2014). Increased heart rate (WebMD, 2014). Feeling tired or weak (WebMD, 2014). Based on this chart, it is clear that some of the signs and symptoms are similar; however, Robert’s family doctor is able to identify some signs that are not objective or noticeable symptoms for Robert, such as the lung crackles and the decreased breath sounds. These signs are primarily associated with defects in the lung. Additionally, I have included some signs and symptoms that are not directly listed in Robert’s case, but are generally present during cases of pneumonia and/or tuberculosis. These signs and symptoms are highlighted in red in the chart above to indicate that they may not necessarily be associated with this specific case. It is important to note that a key history finding is that Robert has emigrated from India about a year ago. This is relevant because India presents a different environment with different potential pathogens than Canada. It is important for the physician to consider this when ordering and evaluating the tests and prescribing any type of medication and treatment. Normal Physiological Function of the Affected Body System Based on the signs and symptoms presented – lung crackles, decreased breath sounds, chronic cough, sputum production – it is likely that the primary body system that is affected is the respiratory system. Specifically, the lungs, which make up the lower respiratory system, are affected. In this section, we will discuss the regular physiological function of the lungs and the entire respiratory system. The respiratory system is made up of four main components: 1) Airways 2) Lungs 3) Blood Vessels 4) Respiratory Muscles (How the Lung Works, 2012). The airways include the following: 1) Nose and linked air passages, which are termed nasal cavities 2) The mouth 3) Larynx 4) Trachea 5) Bronchial tubes and the resulting bronchial branches (How the Lung Works, 2012). During inhalation, air enters the mouth and flows to the trachea. The trachea splits into two bronchi, which enter the lungs (CDC, 2014). It is important to note that the bronchi will branch into conducting and respiratory bronchioles, which eventually terminate in alveolar sacs in the lungs, where gas exchange takes place (CDC, 2014). These airways facilitate the delivery of fresh air from the atmosphere through the trachea and bronchioles to the alveolar sacs (Figure 2) (CDC, 2014). This continuous delivery and removal of air allows the process of gas exchange to take place at the lungs, so that oxygen can be transported into the blood and carbon dioxide can be transported from the blood into the lungs and expired out into the atmosphere (CDC, 2014). It is also important to note that the airways are composed of a mucous epithelium, which heats and humidifies inspired air (How the Lung Works, 2012). This contributes to gas exchange at the alveolar sacs. Additionally, the mucous epithelium of the bronchial and tracheal tubes contains small hair-like particles, called cilia, which can trap and eliminate pathogens and foreign particles (How the Lung Works, 2012). This forms a component of the anatomical immune system. Figure 2: A diagrammatical representation of the respiratory system. Fresh air will enter the mouth into the upper airways, which is composed of the larynx and the trachea. Air will then move into the lower airways, which are composed of the bronchus and bronchioles. Air will finally terminate in the alveolar sacs, which are found in both the left and right lung. Gas exchange takes place at the alveoli (CDC, 2014). As mentioned above, bronchioles terminate in small round air sacs called alveoli. These alveoli are situated in both the right and left lungs. They are covered in a mesh of blood vessels, called capillaries (CDC, 2014). These capillaries connect the pulmonary arteries coming from the right heart carrying deoxygenated blood to pulmonary veins, which carry oxygenated blood back to the left heart, so it can be pumped out to the rest of the body (CDC, 2014). At the capillaries, carbon dioxide from the deoxygenated blood in the pulmonary arteries diffuses into the alveolar sacs and oxygen diffuses from the alveoli into the blood vessels (CDC, 2014). This functions to oxygenate the blood that is travelling back to the heart, so that it can sufficiently oxygenate tissues when it is pumped out to the rest of the body (CDC, 2014). Following gas exchange, exhalation results in the expiration of carbon dioxide rich air. Figure 3: This represents an alveoli situated adjacent to a capillary. There is a thin airblood barrier, which separates the blood in the capillary from the air in the alveolus. Oxygen and carbon dioxide are able to diffuse across this barrier. This diffusion process is termed gas exchange. This process is essential for all tissues of the body because they require oxygen for their normal metabolic functions. Dysfunctions in this gas exchange, as seen in pneumonia and tuberculosis, result in severe deficiencies in blood oxygenation (CDC, 2014). The final component of the respiratory system, the respiratory muscles, are responsible for generating the necessary thoracic pressures, which allow inspiration and expiration to take place (CDC, 2014). These muscles include the diaphragm, intercostal muscles, abdominal muscles and accessory muscles in the neck and collarbone area (Figure 4) (CDC, 2014). The diaphragm is the primary muscle used in inspiration. Its contraction generates the necessary negative thoracic pressure that allows air to flow into the airways and the alveolar sacs (CDC, 2014). During normal breathing, expiration is passive because the relaxation of the diaphragm and the accessory muscles returns respiratory pressures to normal levels; however, during rapid breathing, abdominal muscles play an important role in active expiration (CDC, 2014). Ultimately, the muscles of the respiratory system play an essential role in gas exchange because they allow for the inspiration of fresh air and the expiration of carbon dioxide rich air. Figure 4: An overview of the respiratory muscles responsible for inspiration and expiration (Respiratory Muscles, 2012). Based on this, it is clear that the airways, lungs, blood vessels and respiratory muscles all play an important role in regulating airflow and coordinating gas exchange between the blood and alveolar sacs. Defects in any of these components results in the dysfunction of the overall respiratory system. In the following section, we will discuss the damage assessed to the lung as a result of pneumonia and tuberculosis and how this affects the normal physiological functioning of the lung. Disruption of Normal Physiological Function of the Respiratory System As mentioned earlier, the bacteria that are the most prevalent causes of pneumonia, S. pneumoniae, are members of the human normal flora and colonize the nasopharynx and other regions of the throat (Todar, 2012). S. pneumoniae and other pneumonia-causing bacteria are able to infect the upper respiratory system and gain access to the lower respiratory system, through inhalation, when specific risk factors are present (Eddy, 2005), including pre-existing infection, smoking, chronic obstructive pulmonary disease and asthma (Nair and Niederman, 2011). Therefore, pneumonia starts off as an upper respiratory inflammatory condition and migrates to the lower respiratory system to the alveolar sacs, which are located in the lung (Nair and Niederman, 2011). As this bacterial infection progresses, both the bacteria and the host immune response contribute to the inflammatory condition of the lung and the steady progression of pneumonia (Nair and Niederman, 2011). This results in a disruption of the normal physiological functioning of the lungs and the entire respiratory system. It is important to note that M. tuberculosis initiates a similar inflammatory response and results in similar changes to the respiratory system’s normal physiological function. Therefore, the following discussion on how S. pneumoniae affects the normal physiological function of the respiratory system is also applicable to the M. tuberculosis infection. As the pneumonia-causing bacteria enter the respiratory system, the body initiates a hyperactivation of the inflammatory immune response against the bacteria (Singh, 2012). Neutrophils, macrophages and epithelial cells of the respiratory system mediate this inflammatory immune response (Singh, 2012). The initiation of the inflammatory immune response requires large quantities of blood to be transported to the site of infection, so that neutrophils, macrophages and other cells of the innate immune system can be mobilized (Singh, 2012). This results in vascular congestion of the blood vessels surrounding the alveoli (Singh, 2012). Considering that the air-blood barrier is thin to allow for sufficient gas exchange, engorgement of the blood vessels allows fluid to move through the blood vessel epithelium and into the alveoli (Steel, 2013). Starling’s forces govern this movement of fluid (Steel, 2013). Ultimately, the inflammatory response results in the lung becoming inflamed and consisting of high levels of bacteria, innate immune cells and fluid (Singh, 2012). This excess amount of fluid will adhere to the lining of the chest wall, the alveoli and the space between the chest wall and alveoli, which is termed the interpleural space (Boehlke, 2015). In addition to vascular engorging, this excess fluid is also secreted by the pleural layer (Boehlke, 2015). The overall accumulation of the fluid results in pleural effusion, which impairs breathing by limiting the expansion of the lungs (Boehlke, 2015). This means that the diaphragm and other muscles of inspiration are unable to expand the thoracic cavity enough to generate the negative pressures that are required for inspiration (CDC, 2014). This is termed ventilatory failure (Boehlke, 2015). This results in the alveoli failing to become sufficiently oxygenated, which impairs gas exchange. Additionally, the accumulation of fluid in the alveoli serves to thicken the blood-air barrier, which impairs the process of gas transport (Figure 5) (Boehlke, 2015). This further limits the ability of the lung to oxygenate the blood (Boehlke, 2015). Finally, the inflammatory immune response also results in the severe inflammation and thickening of the walls of the lung and bronchial tubes (Boehlke, 2015). This results in hypoxemic respiratory failure through bronchoconstriction, which limits airflow into the alveolar sacs for gas exchange (Boehlke, 2015). Combining this with the fact that the inspiratory respiratory muscles are impaired and that the blood-gas barrier impairs gas exchange, there are severe limitations in blood oxygenation and carbon dioxide elimination due to the inflammatory immune response. Figure 5: This shows how the alveoli are specifically affected during pneumonia. They are filled with fluid, which prevents normal gas exchange and also has mechanical effects on ventilation (Pneumonia, 2016). Ultimately, the complications of pneumonia including ventilatory failure, hypoxemic respiratory failure and pleural effusion are associated with the symptoms that Robert experiences and the symptoms that are associated with pneumonia and tuberculosis in general. The following is a description of how some of the pathophysiological complications correlate with the symptoms of pneumonia: 1) Fever, chills and night sweats: These symptoms are associated with the general inflammatory immune response. They are mediated by inflammatory substances, such as cytokines in response to both the S. pneumoniae and the M. tuberculosis infections (WebMD, 2014). 2) Chronic productive cough with mucous/sputum and possibly some blood: The cough and the resulting sputum is also a result of the innate inflammatory response (WebMD, 2014). Sputum results because there is an upregulation of mucous generated by the mucous epithelium to eliminate the infection. Blood may also be coughed out because of the damage to the airway epithelium by the inflammatory immune response (Singh, 2012). 3) Shortness of breath: Considering that the airways are constricted and the blood is not sufficiently oxygenated due to the impaired air-blood barrier, there is a sensation of a shortness of breath even though ventilation rate and depth of breathing may be adequate. Shortness of breath is termed dyspnea and it is sensed by chest wall mechanoreceptors, chemoreceptors, lung receptors, and central nervous system mechanisms (Burki and Lee, 2010). These mechanisms stimulate the sense of dyspnea. 4) Increased heart rate: During pneumonia, blood may not be sufficiently oxygenated, so heart rate must increase to mediate the sufficient delivery of oxygen to tissues for essential metabolic functions (WebMD, 2014). 5) Feeling weak or tired: Again, the insufficient oxygenation of blood prevents tissues from being sufficiently oxygenated. As a result, the patient feels weak and tired (WebMD, 2014). 6) Crackles in the lung: This is due to a build up of fluid in the lung, as a result of the inflammatory immune response (LiveStrong, 2015). 7) Decreased breath sounds: Again, this is a result of the fluid accumulation in the lungs (MedlinePlus, 2015). Secondary Sites of Infection Considering that S. pneumoniae and M. tuberculosis are the most common bacterial pathogens associated with pneumonia and tuberculosis, we will consider their ability to spread from the lungs to secondary sites of infection. Although Robert does not display any signs of a secondary infection, as his symptoms are limited to defects in the respiratory system, S. pneumoniae and M. tuberculosis are certainly able to spread to secondary sites. For S. pneumoniae, the secondary sites of infection include the following: 1) Ear: This results in otitis media, which is an ear infection (Todar, 2012). 2) Sinuses: This results in sinusitis, which is an infection of the sinus (Todar, 2012). 3) Peritoneum: This results in peritonitis, which is an infection of the thin tissue, which lines the walls of the abdomen (Todar, 2012). 4) Central Nervous System: This results in meningitis, which is an infection of the brain and spine (Todar, 2012). Various factors are important in the ability of S. Pneumoniae to travel to these secondary sites and infect these areas (Figure 6). The first step in this process is initiating the respiratory infection. This is done through the following steps: 1) The bacteria adhere to the mucosal epithelium of the nasopharynx (Koedel, Scheld and Pfister, 2002). S. pneumoniae express various surface molecules which help them facilitate this adherence. It has been shown that pili proteins facilitate this initial attachment of bacterial cells to the mucosal epithelium (Todar, 2012). Additionally, specific surface proteins, called choline-binding proteins (CBPs) also help to facilitate this attachment (Todar, 2012). 2) Following successful infection of the mucosal epithelium and spread into the lower respiratory system, the bacteria are able to gain entry into the blood by invading the mucous epithelium (Koedel, Scheld and Pfister, 2002). They are then able to initiate bacteraemic spread (Koedel, Scheld and Pfister, 2002). The ability of S. pneumoniae to infect and survive in the blood is crucial to the development of infections in secondary sites because blood provides a route of travel between these sites. The survival of these bacteria in the blood is aided by the presence of a capsular polysaccharide, which prevents phagocytosis by interfering with the biding to complement C3b to the bacterial surface (Todar, 2012). Additionally, the bacteria also produce IgA protease, which cleaves IgA antibodies (Todar, 2012). These structures and proteins allow the bacteria to successfully evade the immune system and enter the secondary sites of infection. 3) Meningitis: The bacteria are able to travel to the CNS, via the blood, and cross the blood-brain barrier into the sterile CNS (Cundell et al., 1995). The blood brain barrier prevents pathogens and other materials from getting into the CNS; however, S. pneumoniae are able to enter the CNS through the brain endothelium (Cundell et al., 1995). To enter the CNS, they must first attach to the endothelial cells (Cundell et al., 1995). Upon contact, S. pneumoniae activate the endothelial cells, which induces their cellular expression of platelet-activating factor (PAF). This binds to bacterial phosphorylcholine expressed on the cell wall (Cundell et al., 1995). Following this, the bacteria are able to enter the CNS and induce inflammatory defenses. The inflammatory immune response is initiated in response to the bacterial DNA, which is released following autolysis, or in response to bacterial cell wall components (Lewis, 2000). Consequently, most of the damage that occurs in the brain in Meningitis is associated with the inflammatory response (Todar, 2012). This damage is induced by the release of host cell proteolytic enzymes, such as matrix metalloproteinases (MMPs), which disrupt the blood-brain barrier, and radical oxygen species, which attack fatty acids and damage neuronal cell membranes (Lukes et al. 1999). This is associated with the loss of brain endothelial cells, which can result in the loss of cerebrovascular autoregulation and loss of blood-brain barrier integrity (Koedel, Scheld and Pfister, 2002). This can lead to severe brain damage and this is the most severe outcome of secondary infection associated with S. pneumoniae (Koedel, Scheld and Pfister, 2002). 4) Other sites of infection: The same characteristics of the bacteria are also important in reaching other secondary sites, including the ear, peritoneum and sinuses (Todar, 2012). These characteristics include their cell wall proteins, such as pili and CBPs, which facilitate their attachment to secondary sites and initiate infection (Todar, 2012). Additionally, the autolysin, LytA, is also important in initiating autolysis of the bacteria, which ultimately induces the inflammatory immune response (Todar, 2012). As mentioned in the case of meningitis, the inflammatory immune response is primarily responsible for inducing the damage at these sites as well; however, the release of hemolysins, such as pneumolysins, also results in bacteria-dependent host cell lysis (Todar, 2012). Additionally, the release of pneumolysins can also induce the further release of cytokines, which contribute to the inflammatory immune response and increase the amount of damage to the site of infection (Todar, 2012). It is important to note that the release of pneumolysins and autolysins also plays a role in the CNS infection (Todar, 2012). Based on this, it is clear that these factors utilized by S. pneumoniae are crucial in establishing the initial infection in the respiratory system, evading the immune response in the blood and establishing a secondary infection at another site, such as the CNS, the ear, sinus or peritoneum. Figure 6: This shows a more extensive overview of how S. pneumoniae spreads to secondary sites and induces infections at those sites (Koedel et al., 2002). Although S. pneumoniae is able to spread beyond the initial infection site and affect secondary infection sites, Robert does not experience any symptoms associated with secondary site infection, which likely means that his infection is isolated to the initial infection site, the lungs. In the case of M. tuberculosis, the secondary sites of infection include the kidney, brain, bones and lymph nodes (Todar, 2012). The bacteria travel to the kidney, brain and bones via the blood and to lymph nodes via the lymphatic system (Todar, 2012). Their ability to travel to these various sites of infection via the blood or lymphatic system depends on their capacity to evade the immune system. They use the following mechanisms to evade the immune system: 1) Intracellular growth: They grow intracellularly in phagosomes, which allows them to evade the immune response (Todar, 2012). Antibodies and complement are ineffective when these bacteria are inside host cells (Todar, 2012). Their ability to grow within phagocytes depends on their capacity to inhibit phagosome-lysosome fusion (Todar, 2012). This is due to a secreted bacterial protein that modifies the phagosome membrane (Todar, 2012). They are also able to inhibit reactive oxygen species, which are essential to the phagocytic process. Enzymes that combat radical oxygen species include AhpC, SodA and SodC (Todar, 2012). 2) Antigen 85 complex: This complex is a secreted group of proteins, which binds fibronectin and walls off the bacteria from the immune response (Todar, 2012). This further facilitates the immune evasion capability of M. tuberculosis. 3) Slow generation time: These bacteria have a slow generation time, which means that the immune system may not recognize them or may not be triggered by them (Todar, 2012). 4) High lipid concentration in cell wall: This high lipid concentration accounts for impermeability and resistance to antimicrobial products (Todar, 2012). It also promotes resistance to osmotic lysis and attack by lysosomes (Todar, 2012). Based on the above factors, it is clear that M. tuberculosis possesses the necessary factors to evade the immune system in the blood and lymphatic system. This allows the bacteria to transit to secondary sites of infection, where they can mediate infection using the following virulence factors: 1) Cell Entry/Adhesion: Once they arrive at these secondary sites of infection, the bacteria are able to adhere and enter host cells, such as macrophages, by binding directly to mannose receptors on macrophages via cell-wall associated mannosylated glycolipid (LAM) (Todar, 2012). Additionally, they are also able to adhere to epithelial cells by utilizing HbhA, which is a surface bacterial protein that binds fibronectin (Todar, 2012). Additionally, M. tuberculosis also utilizes pili proteins to facilitate adherence (Todar, 2012). 2) 19-kDA protein: Once the bacteria are internalized into macrophages at these secondary sites, 19-kDA, which is present on the cell wall, binds to TLR-2 (Todar, 2012). TLR-2 stimulates signaling events, which results in the activation of neutrophils (Todar, 2012). Consequently, the inflammatory immune response is initiated, which facilitates much of the damage to these secondary sites. 3) Cord Factor: This is found on the cell wall and forms a toxic factor to macrophages and other host cells upon initial binding (Todar, 2012). These virulence factors allow M. tuberculosis to bind to phagocytic and epithelial cells and initiate the inflammatory immune response (Todar, 2012). The inflammatory immune response, associated with neutrophil recruitment and cell-mediated hypersensitivity are responsible for most of the damage induced by the bacteria (Todar, 2012). These immune responses are responsible for most of the symptoms seen at the secondary sites of infection. In addition, substances on the bacteria, such as Cord factor, will mediate direct damage to these sites as well (Todar, 2012). Based on this, it is clear that M. tuberculosis has the ability to evade the immune system, which allows it to reach secondary sites of infection through the blood or lymphatic system (Todar, 2012). Additionally, it also has the ability to adhere to specific host cells, initiate the inflammatory and hypersensitive immune response and directly damage host cells through the release of various substances (Todar, 2012). These factors allow the bacteria to reach the secondary sites of infection and damage these sites. It is important to note that the lung can also be a secondary site of infection (Todar, 2012). This occurs when the bacteria return to the lung from the secondary sites of infection and reinitiate the infection years or even decades after the initial infection (Todar, 2012). This shows the persistent nature of M. tuberculosis (Todar, 2012). This persistent nature correlates with the signs the doctor notices and the symptoms that Robert experiences. For example, his chronic productive cough can be explained by the fact that the bacteria are persistent and difficult to eliminate. This is also associated with the fact that the bacteria is intracellular, so it is difficult for the immune response to combat, which results in a chronic infection. Fortunately for Robert, he does not experience any symptoms at the secondary sites, which likely means that the bacteria have not spread beyond the initial infection site of the lungs. References Boehlke, J. (2015). What are Pneumonia Complications? Retrieved from http://www.livestrong.com/article/24987-pneumonia-complications/. Burki, N. K., & Lee, L.-Y. (2010). Mechanisms of Dyspnea. Chest, 138(5), 1196–1201. http://doi.org/10.1378/chest.10-0534 CDC. (2014, June 6). Retrieved from http://www.cdc.gov/niosh/docs/2004-154c/. Cundell, D. R., Gerard, N. P., Gerard, C., Idanpaan-Heikkila, I., & Tuomanen, E. I. (1995). Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature, 377(6548), 435-438. Eddy, O. L. (2005). Community-acquired pneumonia: From common pathogens to emerging resistance. Emergency Medicine Practice, 7(12), 1-24. How the Lung Works. (2012, July 17). Retrieved February 24, 2016 from http://www.nhlbi.nih.gov/health/health-topics/topics/hlw. Koedel, U., Scheld, W. M., & Pfister, H. W. (2002). Pathogenesis and pathophysiology of pneumococcal meningitis. The Lancet infectious diseases, 2(12), 721-736. Lewis, K. (2000). Programmed death in bacteria. Microbiology and Molecular Biology Reviews, 64(3), 503-514. LiveStrong. (2015, April 15). Reasons for Crackling in the Lungs. Retrieved February 23, 2016 from http://www.livestrong.com/article/123656-reasons-crackling-lungs/. Lukes, A., Mun-Bryce, S., Lukes, M., & Rosenberg, G. A. (1999). Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Molecular neurobiology, 19(3), 267-284. MayoClinic. (2015, March 14). Pneumonia. Retrieved February 23, 2016 from http://www.mayoclinic.org/diseases-conditions/pneumonia/basics/definition/con20020032. MedlinePlus. (2015, March 5). Breath Sounds. Retrieved February 23, 2016 from https://www.nlm.nih.gov/medlineplus/ency/article/007535.htm. Nair, G. B., & Niederman, M. S. (2011). Community-acquired pneumonia: an unfinished battle. Medical Clinics of North America, 95(6), 1143-1161. Niamh, K., & Lowe, C. (2016). In Signs and Symptoms. Retrieved from https://connect.ubc.ca/webapps/blackboard/execute/content/blankPage?cmd=view &content_id=_3233187_1&course_id=_68245_1&mode=reset Pneumonia. (2016). Retrieved from http://medicalassessmentonline.com/terms.php?R=323&L=L. Respiratory Muscles. (2012). Pearson Education. Retrieved from http://www.slideshare.net/TheSlaps/dr-b-ch-24lecturepresentation. Singh, Y. D. (2012). Pathophysiology of community acquired pneumonia. The Journal of the Association of Physicians of India, 60, 7-9. Steel, H. C., Cockeran, R., Anderson, R., & Feldman, C. (2013). Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediators of inflammation, 2013. Todar. (2012). Todar’s Online Textbook of Bacteriology. Retrieved from http://textbookofbacteriology.net/S.pneumoniae.html. Tuberculosis. (2016, February 23). Retrieved from http://www.mayoclinic.org/diseasesconditions/tuberculosis/home/ovc-20188556. WebMD. (2014, September 9). Pneumonia. Retrieved February 23, 2016 from http://www.webmd.com/lung/tc/pneumonia-related-information.