* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download BIOL 103 Global climate change science
General circulation model wikipedia , lookup
Fossil fuel phase-out wikipedia , lookup
Climate engineering wikipedia , lookup
2009 United Nations Climate Change Conference wikipedia , lookup
Global warming hiatus wikipedia , lookup
Fred Singer wikipedia , lookup
Instrumental temperature record wikipedia , lookup
Attribution of recent climate change wikipedia , lookup
Public opinion on global warming wikipedia , lookup
Low-carbon economy wikipedia , lookup
United Nations Climate Change conference wikipedia , lookup
United Nations Framework Convention on Climate Change wikipedia , lookup
Climate change in the United States wikipedia , lookup
Climate change mitigation wikipedia , lookup
Climate change in Canada wikipedia , lookup
Years of Living Dangerously wikipedia , lookup
Global warming wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
IPCC Fourth Assessment Report wikipedia , lookup
Business action on climate change wikipedia , lookup
Greenhouse gas wikipedia , lookup
Politics of global warming wikipedia , lookup
Mitigation of global warming in Australia wikipedia , lookup
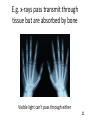
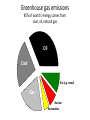
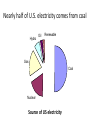
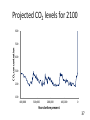
BIOL 103 Global climate change science Warning: You will need to be able to describe the Keeling Curve to pass this course 2 Lecture outline 1. The “greenhouse effect” 2. Anthropogenic greenhouse emissions 3. Changes to the atmosphere 4. Changing climate 5. How to study climate 3 Let’s start by discussing this diagram in more detail 4 A primer on radiation: Sunlight is a mixture of different types of radiation 5 We can think of light as small packets of energy (called photons) traveling in a wave Photon Photons are not matter or atoms (Which was why plants can’t use as a raw material 6 Colors are radiation with different wavelengths 7 Radiation with wavelength shorter than violet or longer than red are invisible to human eye Ultraviolet radiation has shorter wavelength than violet Infrared radiation has longer wavelength than red 8 Electromagnetic radiation: Particles of energy traveling at speed of light in waves Wavelength determines properties Notice: Gamma rays, x-rays, ultraviolet radiation, visible light, infrared radiation, microwaves, and radio waves differ only in their wavelength 9 When radiation strikes something, it can do three things: Reflect: Bounce off Absorb: Energy taken up by material Transmit: Pass through 10 E.g. x-rays pass transmit through tissue but are absorbed by bone Visible light can’t pass through either 11 Sunlight hitting atmosphere will either reflect, transmit, or absorb About half of sunlight is absorbed by the ground 12 What happens to sunlight after being absorbed? • It doesn’t disappear. • Energy never disappears. • It causes ground to warm up. • If this was the entire story, earth would get warmer and warmer (forever). • However, earth radiates this energy back to space. • This radiation is called infra-red radiation. 13 Infra-red radiation Radiation with longer wavelength than light We perceive infra-red radiation as heat We are radiating infra-red radiation constantly 14 Earth’s atmosphere absorbs infra-red radiation more than sunlight This causes greenhouse effect. 15 Summary of greenhouse effect 1. Sunlight passes through atmosphere. 2. Sunlight absorbed by earth. 3. Earth emits infra-red radiation. 4. Infra-red radiation absorbed by atmosphere (warming atmosphere) 16 Composition of Earth’s Atmosphere Nitrogen Oxygen … Carbon dioxide … Methane 78% 21% 0.03% 0.002% 17 Several types of “greenhouse” gases trap heat Gas Relative heat trapping ability Carbon dioxide 1 Methane 23x* Nitrous oxide 296x Hydrochlorofluorocarbons 12,000x *Indicates each methane molecule traps 23x as much heat as each CO2 molecule. 18 The natural greenhouse effects warms earth 59°F Make life as we know it possible. 19 2. Human populations have grown rapidly Population 6 Air pollution Water pollution Soil erosion Overfishing Deforestation 4 2 0 0 500 1000 Year 1500 2000 20 Burning coal, oil, or natural gas releases CO2 to the atmosphere 21 A useful word: Anthropogenic (adj) Caused by humans 22 Greenhouse gas emissions 85% of world’s energy comes from coal, oil, natural gas Oil Coal Bio (e.g. wood) Gas Nuclear Renewables Nearly half of U.S. electricity comes from coal Hydro Oil Renewable Gas Coal Nuclear Source of US electricity Electricity production is largest source of CO2 emissions in the United States 50% of electricity is produced by burning coal. 20% produced by burning natural gas. Both release CO2. 25 Transportation is second largest source of emissions 26 There are 136,000,000 passenger cars and 99,000,000 SUVs and light trucks in the United States 27 Recall: A Subaru Forester wagon releases almost 7 tons of CO2 per year! 386 grams / mile × 15,000 miles = 5,790,000 grams = 12,764 pounds ~ 6 ¾ tons of CO2 per year! *Source: www.fueleconomy.gov 28 Global CO2 emissions from fossil fuels 62,000,000,000,000 (62 trillion) pounds Or 31 billion tons Or 31 gigatons 29 Deforestation is another major source of CO2 emissions 30 Even cow farts are a significant source of greenhouse gases (methane) 100 million cattle in U.S. 1.2 billion cattle, sheep, goats worldwide Methane emissions being collected from a cow. 31 32 3. Changes to global atmosphere CO2 levels are now being measured around the world 33 CO2 levels are gradually increasing CO2 Concentration (ppm) 400 390 380 Highest levels in past 650,000 years 370 360 350 340 330 CO2 levels will probably double this century 320 310 300 1960 1970 1980 Year 1990 2000 34 390 388 CO2 Concentration (ppm) 386 384 382 380 378 Annual increase caused by fossil fuel emissions 376 374 372 370 2004 2005 2006 Year 2007 35 Global CO2 levels from Antarctic ice cores 350 CO2 concentration 300 250 200 150 Ice Ages 100 400,000 300,000 200,000 100,000 0 Years before present 36 Projected CO2 levels for 2100 600 CO2 concentration 500 400 300 200 100 400,000 300,000 200,000 100,000 0 Years before present 37 Carbon dioxide, Methane, Nitrous oxide levels have all risen dramatically over past 10,000 years Graphs show 10,000 years of data 38 Who put the extra CO2 in the atmosphere 39 China is world’s largest CO2 emitter United States emits most per capita 40 Not all CO2 emissions end up in atmosphere Where does CO2 go? 40% atmosphere 30% forests (increased tree growth) 30% oceans (dissolved) (These numbers are approximate and are poorly known. This is an active area of research.) 41 Greenhouse gases won’t escape out the ozone hole The ozone hole is not similar to a hole in an air mattress. It is a reduction in a gas that makes up a tiny fraction of the atmosphere. 42 Nor does ozone hole “let in” sunlight Note: CFCs’ and ozone molecules do have a small impact on global warming. But for all practical purposes, global warming is unrelated to the 43 ozone hole. 4. Global warming Swedish physicist predicted in 1896 that doubling CO2 would raise earth’s temperature by 9°F Svante Arrhenius Arrhenius predicted burning fossil fuels would take 3000 years to double CO2 levels. Viewed warming as a good thing - avoid ice age, help feed planet. 44 Length of glacier 1 unit = 1km Glaciers have been retreating all around the world Year 45 “The Hockey Stick Graph*” Average global temperature rose 1°F last century 46 *This is an important graph. Amount of warming in 21st century will depend on many social factors • Human population growth • Rate at which wind, solar, nuclear power replace fossil fuels • Energy efficiency • Urban sprawl & public transportation • Industrialization in the developing world (esp. India & China) • Deforestation in the Amazon • How much meat people eat 47 Best estimate: 5°F of warming Graph shows projections for different scenarios 48 Does this mean a 5°F warming is inconsequential? 100 Temperature 80 60 40 20 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Jan 49 No. Ice ages were only 8°F cooler! 50 Study guide • Read Chapter 18, “Global climate change,” thoroughly. (It is the basis for 3 lectures.) • Be able to describe the greenhouse effect. • Be able to identify how much the planet warmed in the 20th century, and how much sea levels rose. • Be able to sketch the “hockey stick graph.” • Be able to describe why CO2 and methane levels in the atmosphere are increasing. • Be able to identify how much CO2 a typical car releases into the atmosphere annually. • Be able to describe two other sources of greenhouse gases besides burning of fossil fuels. • Be able to discuss how US and China CO2 emissions compare. • Be able to identify which countries have released the most CO2 into the atmosphere. • Review “Baloney detection kit” from lecture 2 and be able to apply it to climate change controversy. • Be able to use all of the above points to evaluate arguments. 51 Sample exam questions How much did the earth warm in the 20th century? How much did sea levels rise in the 20th century. Use a diagram to explain the greenhouse effect. Evaluate the following statement: “Because industrialized countries burn the most fossil fuels, they are only countries that will be able to prevent global warming in the 21st century.” 52 The end 53