* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Answers / Solutions
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Aromatization wikipedia , lookup
Petasis reaction wikipedia , lookup
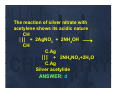
HYDROCARBONS I & II PHENOLS ALCOHOLS & ETHERS 1. During electrophilic substitution of benzene the intermediate species benzene, involved is a) Carbanion b) Carbocation c)) Free radical d)) none of these Carbocation, since benzene is electron rich ANSWER: b 2 In the reaction 2. FeCl3 C6H6 + Cl2 ⎯⎯⎯→ C6H5Cl + HCl ( chlorobenzene) The attacking reagent is a) Cl2 b) Cl+ c) Cl d) FeCl4 Chorination of benzene takes place through the attack by Cl+ (chloronium ion) ANSWER: b 3. Methyl bromide when heated with zinc in a closed tube produces a) methane b) ethane c)) ethyne th d) methanol th l This iis a W Thi Wurtz t type t reaction. ti Instead of sodium, other metals such as Ag, Cu, Zn can also be used in finely divided state. ANSWER: b 4. During g the preparation of ethane by y Kolbe’s electrolytic method using inert electrodes the pH of the electrolyte a) increases progressively as the reaction proceeds b) decreases progressively as the reaction ti proceeds d c) remains constant throughout the reaction d) may decrease if the concentration of the electrolyte is not very high During D i the th preparation ti off ethane th by b Kolbe’s electrolytic method using inert electrodes the pH of the electrolyte 2CH3COONa COONa+ 2H2O O⎯Electrolysis Electrolysis → CH3 – CH3 + 2CO2 + H2 + 2NaOH Since NaOH is formed, the pH increases p As the proceeds. ANSWER: a 5. Nitrobenzene can be prepared from benzene by using a mixture of conc. HNO3 and conc. conc H2SO4 In the nitrating mixture, HNO3 acts as a a) base b) acid c) reducing agent d) catalyst. HNO3 acts as base as it provides–OH¯ H2SO4 ⎯→H →H+ + HSO4¯ O2N – OH + H+ ⎯⎯⎯→NO2+ + H2O ANSWER: a 6 When 6. Wh a mixture i t off methane th and d oxygen is passed through heated molybdenum l bd oxide, id the th main i product formed is a)) methanoic th i acid id b) ethanal th l c) methanol d) methanal MoO CH4 + O 2 ⎯⎯→ HCHO + H2O 550K ANSWER: d 7. which of the following can’t be used in Friedal-Crafts Friedal Crafts reactions? a) FeCl3 b) BF3 c) AlCl3 d) NaCl Friedal-Crafts Friedal Crafts alkylation reaction require Lewis acid catalysts and but NaCl is not a Lewis acid acid. ANSWER: d 8. Which one of the following has the minimum boiling point? a) n-Butane b) Isobutane c) 1-Butene d) 1- Butyne Boiling points follow the order alkyne > alkene > n-alkane > branched alkane alkane. ANSWER: b 9 On mixing certain alkane with chlorine 9. and irradiating it with ultraviolet light, it forms only one monochloroalkane. The alkane is: a) isopentane b) neopentane c)) p propane p d)) p pentane Neopentane (CH3)4C is a symmetrical alkane lk and d gives i only l one monoalkane derivative. ANSWER: b 10. Pure methane can be produced by a) Wurtz reaction b) Kolbe’s electrolytic method c)) Soda-lime S d li d decarboxylation b l ti d) reducetion with H2 sodalime CH3COONa COON + NaOH N OH ⎯⎯→ CH4+ Na N 2CO3 ∆ ANSWER: c 11 Which of the following species 11. participate in sulphonation of b benzene ring? i ? a) H2SO4 b) HSO3 c) SO3 d) SO2 In sulphonation of benzene, the species which attack benzene ring is SO3 ANSWER : c 12. A gas decolourises alkaline KM O4 solution KMnO l ti but b t does d nott give precipitate g p p with AgNO g 3. It is a) CH4 b) C2H4 c) C2H2 d) C2H6 ANSWER: b As C2H4 (alkene) is not acidic in nature therefore, nature, therefore it does not give any precipitate with AgNO3. However it is oxidized with dilute However, alkaline KMnO4 (Baeyer’s reagent) to glycol CH2 Alk KMnO4 Alk.KMnO + H2O + O ⎯⎯⎯⎯→ CH2 CH2OH ⎪ CH2OH 13. In its reaction with silver nitrate it t acetylene t l shows h a) b)) c) d) Oxidising property Reducing g property p p y Basic proerty Acidic property The reaction of silver nitrate with acetylene shows its acidic nature CH ⎪⎪⎪ + 2AgNO3 + 2NH4OH ⎯→ → CH C Ag C.Ag ⎪⎪⎪ + 2NH4NO3+2H2O C Ag C.Ag Silver acetylide ANSWER d ANSWER: 14. Benzene is obtained by fractional distillation of a) Heavy oil b) Anthracene oil c) Middle oil d) Light oil Benzene is obtained by the Fractional distillation of light oil. oil Answer: d 15 The function of AlCl3 in Friedal 15. Craft’s reaction is a) to absorb water b) to absorb HCl c) to produce electrophile d) to produce nucleophile AlCl3 produces attacking electophile as follows o o s AlCl C 3+Cl-Cl C C ⎯⎯→ → AlCl C 4+ C Cl+ ANSWER: c 16 Benzene 16. B d does nott undergo d addition reaction easily because a)) It has h a cyclic li structure t t b) Double bonds in it are very strong c)) Resonance R stabilised t bili d system t iis to t be preserved d) It has h six i hydrogen h d atoms t Benzene is resonance stabilised and as such resistant to addition reactions. ANSWER: c 17. The presence of unsaturation in 17 organic compounds can be tested with a) Schiff Schiff’s s reagent b) Tollen’s reagent c)) Fehling’s F hli ’ reagentt d) Baeyer’s reagent. Presence of unsaturation in organic compounds can be tested with Baeyer Baeyer’s s reagent. ANSWER d ANSWER: 18. Reactions of alkanes with halogens is explosive in case of a) F2 b) Cl2 c) Br2 d) I2 Reaction of alkanes with F2 is explosive. ANSWER: a 19. Most common reactions of benzene and its derivatives are a) Elecrophilic addition reactions b) Electrophilic stustitution reactions c) Nucleophilic addition reactions d) Nucleophilic substitution reactions. Benzene give electrophilic substitution reactions. ANSWER: b 20 The most reactive hydrocarbon is 20. a) Ethene b) Ethyne c) Ethane d) Methane Alkynes are less reactive than alkenes. ANSWER: a 21 To a mixture of fuming HNO3 and 21. conc. H2SO4, benzene was added. This mixture was heated for f long time at 100°C. The main product is a) C6H5NO2 b) C6H5SO3H c) 1,3,5-trinitrobenzene d) m-Dinitrobenezene m Dinitrobenezene ANSWER: c 22 Vulcanized 22. V l i d rubber bb resists i t a) Jerking motion b) cold temperature c)) chemical corrosion d) wear and tear due to friction. Vulcanized rubber has lot of cross bonding between polymer chains by sulphur cross links links. Hence it becomes strong and resistant to wear and tear. ANSWER d ANSWER: 23. In Buna-S, the symbol Bu stands for a) 1-Butene b) n-Butane c) 2-Butene d) Butadiene In Buna-s,, Bu stands for butadiene and S stands for styrene. ANSWER: d 24 The catalyst used in the manufacture 24. of polyethene by Zeigler method is a)) Titanium Tit i tetrachloride t t hl id and d triphenyl aluminium b) Titanium tetrachloride and triethyl aluminium c) Titanium dioxide d)) Titanium isoperoxide p ANSWER: b 25. Which is most strained cycloalkane? y a) Cyclohexane b) Cyclopropane C l c) Cyclobutane d) Cycloctane ANSWER: b 26 Most stable conformation of 26. cyclohexane is a) Planar chair conformation b)) Non p planar chair conformation c) Planar boar conformation d) Non planar board conformation. conformation To minimize the angle strain cyclohexane assumes two non planar structures-Boat and Chair forms. In chair form there is least repulstion. Hence most preferred conformation of cyclohexane is chair conformation which is non planer. ANSWER: b 27 According to Baeyer 27. Baeyer’s s strain theory which among the f ll i is following i highly hi hl stable? t bl ? a) Cyclohexane b) Cycloheptane c) Cyclopentane d) None of these Angle stain in cyclopentane is minimum and hence is highly stable. ANSWER c ANSWER: 28. Which of the following reagents g can react with phenol p to produce phenolphthalein ? a) phthalic anhydride / H2SO4 b) ethanoic anhydride / H2SO4 c) CO2, CCl4 d) Potassium phthalimide Phthalian fusion test ANSWER: a 29. The most acidic compound among g the following g is a) phenol b) m m-cresol cresol c) p-Nitrophenol d) Picric acid Picric acid ANSWER: d 30. Identify y the p product Z in the following sequence of reaction NaOH Phenol ⎯⎯⎯→ X CO2, 4 4-7atm, 7atm, ⎯⎯⎯→ 410 K H3O + Y ⎯⎯⎯→ Z a) aspirin b) salicylaldehyde c) benzoic acid d) salicylic acid acid. Kolbe’s Schmidt reaction. ANSWER d ANSWER: 31 Phenol, 31. Phenol p-Methyphenol, p Methyphenol m-Nitrophenol m Nitrophenol and p-Nitrophenol follows order of increasing acidic strength a) Phenol, p-Methylphenol, p-Nitrophenol, m-Nitrophenol b) p-Methylphenol, Phenol, m-Nitrophenol, p p-Nitrophenol, p , c) p-Methylphenol, m-Nitrophenol,Phenol, p-Nitrophenol, d) m-Nitrophenol Nit h l p-Nitrophenol, Nit h l Phenol, Ph l p-Methylphenol, ANSWER: b. 32. Inorder to get Bakelite from phenol which of the following p g reagent is required? a) b)) c) d)) HCHO CHCl3 / NaOH CCl4 / NaOH HCHO / H+ or OH HCHO / H+ or OH ANSWER: d 33. Phenol is more readily soluble in a) dil. HCl b) Both B th NaOH N OH and d HCl c) NaOH sol d) Sodium Sodi m bicarbonate solution. sol tion since it is very weak acid is soluble in NaOH solution. ANSWER: c 34 Carbolic acid is 34. a)) aqueous q solution of phenol p b) phenyl benzene c) phenyl acetate d) Salol aqueous solution l ti off phenol h l ANSWER: a 35. Organic acid without a carboxylic ca bo y c g group oup is s a) b) c) d) ascorbic acid vinegar i oxalic acid picric i i acid id Picric acid is 2 2,4,6-Trinitrophenol. 4 6-Trinitrophenol ANSWER: d 36 Salicylaldehyde can be prepared 36. from a) Phenol and chloroform b) Phenol, chloroform and sodium hydroxide c) Phenol, carbon tetrachloride and NaOH d) None Phenol, Ph l chloroform hl f and d sodium di hydroxide , Reimer – Tiemann’s reaction. ANSWER: b 37 Phenol is treated with bromine water 37. and shaken well to get white precipitate. i it t The Th white hit precipitate i it t is i a) 1-Bromophenol b) 2,4,6-Tribromophenol 2 4 6 T ib h l c) 2,4-Dibromophenol d) Mixture of o- and p-bromophenol Bromination of phenol in aqueous medium gives 2,4,6-Tribromophenol. ANSWER: b 38. Cumene is the compound used for commercial preparation of phenol. Chemically cumene is a) Isopropyl benzene b) ethyl benzene c) n-propylbenzene n propylbenzene d) None of above isopropyl benzene ANSWER: a 39. Hybrid state of central oxygen atom in ether is a) 2 sp c) sp ANSWER : b b) 3 sp d) sp3d 40 Oxygen atom in ether is : 40. a) b) c) d) very active replaceable active comparatively inert. The divalent oxygen is linked g y to C–atoms on both sides strongly and there are no active sites like OH, C=O etc. in it. ANSWER: d 41. Which of the following p is used as compounds an anesthesia? a) b) c)) d) ethyl alcohol acetic acid di th l ether diethyl th acetic anhydride ANSWER: c 42. When diethyl ether is treated with excess of Cl2 in the presence of sunlight, sunlight the product formed is : a) CH3CHCl–O–CH2CH3 b) CH3CHCl CHCl–O–CHClCH O CHClCH3 c) CCl3CCl2–O–CCl2CCl3 d) CH3CCl2 –O– CHClCH3. ANSWER: c ANSWER 43. An ether is more volatile than an alcohol having the same molecular formula. This is due to a) b) c) d) dipole character of ethers alcohols having resonance structure inter-molecular hydrogen bonding in ethers inter- molecular hydrogen bonding in alcohols. Alcohols have stronger g inter molecular bonding ( H-bonding) and d ethers th have h weaker k intermolecular bonding (vander Waals). ANSWER: d 44. All alcohols are a) b) c) d) completely soluble in water ionized in water not soluble in water soluble organic solvents All alcohols are not soluble in water. Alcohols with large size of RR are almost immiscible with water. ANSWER: c 45. Power alcohol is a mixture of petrol and alcohol in the ratio of a)) 4:1 b) 1 : 4 c) 2:1 d) 1 : 2 4 : 1 ( 80% petrol and 20 % alcohol) ANSWER: a 46. Widespread deaths due to liquor poisoning is because of a) b) c) d) presence of bad compound in liquor presence of methyl alcohol presence of ethyl alcohol presence of carbonic acid. ANSWER: b 47. The dehydration of butane–1–ol gives a) b) c) 1 – butene as the main product 2 – butene as the main product equal amounts of 1 – butene and 2 – butane d) 2 – methyl propene. propene The dehydration of 1–butanol gives 2 b t 2–butene as the th main i product d t because 2° carbocation is stabler than 1° ANSWER b ANSWER: 48. Rectified spirit can be converted into absolute alcohol by a) Fractional distillation b) steam distillation c)) cannott be b converted t d d) by putting rectified spirit in contact with quick lime followed by y distillation. Quick lime has stronger affinity for water. ANSWER: d 49. Which one is primary alcohol ? a) b) c) d) Buten –2–ol propan–2–ol Butan–1– ol 2,3–Dimethylhexane–4–ol. H3C–CH2–CH2–CH2–OH Butan–1–ol. ANSWER: c 50 In reaction of alcohols with alkali 50. metal, which of the following alcohol will react fastest ? a) secondary b) tertiary c) primary d) all equal Primary alcohol because the reaction Involves cleavage of strongest acidic group (O – H) of various types of alcohols alcohols. ANSWER: c 51. Primary and secondary alcohols on action of red hot copper give a) Aldehydes and ketones respectively b) Ketones and aldehydes respectively c)) Only O l aldehydes ld h d d) Only ketones. 51. Primary and secondary alcohols on action of red hot copper give RCH2OH with Cu at 573K gives RCHO and R2CHOH gives R2CO ANSWER: a