* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The DUET gene is necessary for chromosome
Gene nomenclature wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Point mutation wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
Genomic imprinting wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Genetically modified organism containment and escape wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
History of genetic engineering wikipedia , lookup
Gene expression profiling wikipedia , lookup
Y chromosome wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Designer baby wikipedia , lookup
Genome (book) wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Gene expression programming wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Microevolution wikipedia , lookup
Neocentromere wikipedia , lookup
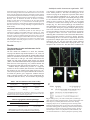
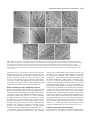
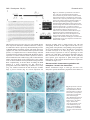
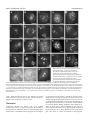
Research article 5975 The DUET gene is necessary for chromosome organization and progression during male meiosis in Arabidopsis and encodes a PHD finger protein Thamalampudi Venkata Reddy1,*, Jagreet Kaur1,*, Bhavna Agashe1,*, Venkatesan Sundaresan2 and Imran Siddiqi1,† 1Centre for Cellular and Molecular Biology, Uppal 2Department of Plant Biology and Agronomy, Life Road, Hyderabad – 500007, India Sciences Addition 1002, University of California, Davis, CA95616, USA *These authors contributed equally to this work †Author for correspondence (e-mail: [email protected]) Accepted 27 August 2003 Development 130, 5975-5987 © 2003 The Company of Biologists Ltd doi:10.1242/dev.00827 Summary Progression through the meiotic cell cycle is an essential part of the developmental program of sporogenesis in plants. The duet mutant of Arabidopsis was identified as a male sterile mutant that lacked pollen and underwent an aberrant male meiosis. Male meiocyte division resulted in the formation of two cells instead of a normal tetrad. In wild type, male meiosis extends across two successive bud positions in an inflorescence whereas in duet, meiotic stages covered three to five bud positions indicating defective progression. Normal microspores were absent in the mutant and the products of the aberrant meiosis were unito tri-nucleate cells that later degenerated, resulting in anthers containing largely empty locules. Defects in male meiotic chromosome organization were observed starting from diplotene and extending to subsequent stages of meiosis. There was an accumulation of meiotic structures at metaphase 1, suggesting an arrest in cell cycle progression. Double mutant analysis revealed interaction with dyad, a mutation causing chromosome cohesion during female meiosis. Cloning and molecular analysis of DUET indicated that it potentially encodes a PHD-finger protein and shows specific expression in male meiocytes. Taken together these data suggest that DUET is required for male meiotic chromosome organization and progression. Introduction al., 1999; Bai et al., 1999; Yang et al., 1999a; Grelon et al., 2001; Armstrong et al., 2002; Chen et al., 2002). Others appear to be unique to plants and do not have obvious homologues in yeast or animals (Byzova et al., 1999; Mercier et al., 2001; Azumi et al., 2002). The pathway leading to sporocyte specification is found only in plants and the two genes that have been identified in this pathway, SPOROCYTELESS/NOZZLE which encodes a nuclear protein related to MADS box transcription factors and is required for the initiation of sporogenesis (Yang et al., 1999b; Schiefthaler et al., 1999) and EXCESS MICROSPOROCYTES1/EXTRA SPOROGENOUS CELLS, which encodes a putative LRR receptor kinase required for tapetum formation and control of male meiocyte number (Canales et al., 2002; Zhao et al., 2002), do not have corresponding homologues in yeast or animals. The control of meiotic cell cycle progression in yeast is dependent upon checkpoints that monitor morphogenesis of the chromosomes during meiosis. Mutations that affect synapsis and recombination lead to arrest of meiotic progression at the pachytene stage. The identification and analysis of extragenic suppressors of pachytene arrest has led to an understanding of the pachytene checkpoint (Roeder and Bailis, 2000). The pachytene checkpoint has also been found in animals (Edelmann et al., 1996), but remains to be identified Meiosis in plants is the transition between the diploid sporophyte and haploid gametophyte generations which in lower plants exist as distinct free-living organisms. During the reproductive phase of development in flowering plants, specialized meiotic cells, the sporocytes, are formed in the anthers and ovules. In plants there is no separate germline as in animals, and the sporocytes are derived from the L2 layer of the meristem (Dawe and Freeling, 1990). The understanding of sporocyte specification and meiosis in plants has advanced significantly in recent years (reviewed by Yang and Sundaresan, 2000; Bhatt et al., 2001). Genetic and molecular approaches in Arabidopsis and maize have led to the isolation of mutants that are altered in sporocyte development or meiosis (Curtis and Doyle, 1991; McCormick, 1993; Chaudhury et al., 1994; Sheridan et al., 1996; Golubovskaya et al., 1997; Ross et al., 1997; Taylor et al., 1998; Sanders et al., 1999) as well as to the identification of several genes that are required for development of the sporocyte or for meiosis. Many of the plant genes that are responsible for basic components of the meiotic machinery that is common to all eukaryotes such as chromosome cohesion, synapsis, recombination and chromosome segregation show conservation with genes in yeast and other organisms (Klimyuk et al., 1997; Couteau et Key words: Chromatin, Male sterility, Checkpoint, Cohesion, Synapsis 5976 Development 130 (24) in plants (Couteau et al., 1999; Garcia et al., 2003). In addition to the pachytene checkpoint, which is specific to meiosis, chromosomal checkpoints that act during mitosis have also been shown to function in meiosis (Lydall et al., 1996). This is consistent with the view that meiosis is a specialized cell cycle based on the mitotic cell cycle (reviewed by Lee and Amon, 2001). There is limited information on the control of meiotic progression in plants. Arabidopsis mutants that affect meiotic progression have been described (Siddiqi et al., 2000; Magnard et al., 2001) and several genes have also been characterized at the molecular level. The ASK1 gene is required for homologue separation during male meiosis (Yang et al., 1999a). The DYAD/SWI1 gene is required for meiotic chromosome organization and meiotic progression (Mercier et al., 2001; Agashe et al., 2002). The Swi1 protein has been shown to be expressed in G1 and S phase of meiosis, and required for axial element formation and initiation of recombination (Mercier et al., 2003). The SOLO DANCERS gene encodes a cyclin-like protein that is required for synapsis during meiosis (Azumi et al., 2002). Several of these genes appear to be associated with changes in chromosome organization and dynamics, however the mechanism by which these changes are related to the progression of meiosis in plants remains unknown. We describe the isolation and characterization of the duet mutant of Arabidopsis and molecular analysis of the DUET gene. We show that the duet mutant is defective in chromosome organization and progression during male meiosis. duet also shows a synergistic genetic interaction with the dyad mutant. The DUET gene encodes a plant homeo domain (PHD)-finger protein that is expressed in male meiocytes. Materials and methods Plant material and growth conditions Arabidopsis growth conditions were as described earlier (Siddiqi et al., 2000). Light microscopy Developmental analysis of whole-mount anthers and ovules was done after clearing inflorescences in methyl benzoate as described previously (Siddiqi et al., 2000). The anthers were dissected on a slide under a stereo dissecting microscope, mounted with a coverslip and observed on a Zeiss Axioplan imaging 2 microscope under DIC optics using ×40 and ×100 oil immersion objectives. Photographs were captured on a CCD camera (Axiocam, Carl Zeiss) using the Axiovision program (version 3.1). For a stage-wise comparison of pollen development in the wild type and the duet mutant, the ovules from the corresponding pistils were staged and used as a reference for pollen developmental stage. Scoring of ovule stages was based on examination of all the ovules in a pistil. The pistil was mounted either intact or after separating the two carpels, and ovules were viewed through the carpel wall. At the early stages of ovule development corresponding to pollen meiosis and gametogenesis, all ovules in a pistil were at the same stage. Anthers were also bissected and developing pollen was observed in optical sections taken through the anther wall. Pollen development in anthers was also found to be synchronous. The correspondence between ovule and pollen stages across different inflorescences was consistent. For plastic sections the inflorescences were fixed in 2% paraformaldehyde, 0.5% glutaraldehyde in 1× PBS overnight at 4°C. The inflorescences were then washed with 1× PBS, treated with osmium tetroxide for 3 hours, followed by dehydration in a graded ethanol series (30%, 50%, 70%, Research article 90%, 100% ×5) for 15 minutes each. Ethanol was replaced by propylene oxide and the samples infiltrated with Araldite resin followed by embedding and curing for 3 days at 60°C. 2 µm sections were cut using a Reichert Ultracut E microtome. Sections were stained with 1% Toluidine Blue in 1% borax for 2-5 minutes and mounted in the Araldite resin. Bright-field photographs of anther cross sections were taken using a Zeiss Axioplan imaging 2 microscope. Photographs were taken on Kodak Supra 100 ISO film using a blue filter. All the photographs were edited using Adobe Photoshop 5. cDNA isolation and expression analysis Poly(A)+ mRNA was isolated from young flower buds and leaves using the PolyA tract mRNA isolation kit (Promega) according to the manufacturer’s protocol with an inclusion of RQ1 DNAase (Promega) treatment before mRNA precipitation. Pistils were dissected and stored in RNA Later (Ambion) and total RNA was isolated using Trizol (Gibco BRL Life Sciences). The cDNA synthesis was carried out with 150 ng of poly(A)+ RNA or 5 µg total RNA in the case of dissected pistils, using the Superscript choice system for cDNA synthesis (Gibco-BRL Life Sciences). Amplification of DUET cDNA was carried out by using the gene-specific primers SETAF (5′CGTCTCCATCGAAGCTAAAATC-3′) and SETR1 (5′-ATCTACAAAGTTTGATCCAAAAACTGAC-3′). The amplified cDNA was cloned into a pMOSBlue Vector (Amersham Pharmacia Biotech) and sequenced. DUET expression was examined by PCR using cDNA prepared from poly(A)+ mRNA as template and the primers SETF1 (5′-CCAATCATCGAAACGTGTCGTAAGAG-3′) and SETR13 (5′TCCGAGACTATTACAAAGCCGATCC-3′). GAPC expression was detected using the primers GAPC1 (5′-CTTGAAGGGTGGTGCCAAGAAGG-3′) and GAPC2 (5′-CCTGTTGTCGCCAACGAAGTCAG-3′). DYAD cDNA was amplified with the gene specific primers 3RR1 (5′-CATGGAAGAGACCTTACCAGTTCACATCA-3′) and g2.2r7 (5′-AGCTAGTGATTATTGGAGAAACCTTGCG-3′). In situ hybridization was carried out as described previously (Siddiqi et al., 2000). A 1.26 kb coding region of DUET cDNA lacking the PHD-finger domain was amplified using the primers STMF1 (5′-GGTCATTTGGTATGTGTCAATGGTATGG-3′) and STMR3 (5′-TCAATCTCAGTCACTACAAAATTTGACAAG-3′) and subcloned into pGEM-T (Promega) for synthesis of RNA probes. Molecular analysis of the duet mutant locus Southern hybridization experiments using a Ds transposon-specific probe derived from pWS31 (Sundaresan et al., 1995) was used to establish copy number of the insertion. TAIL-PCR was used to amplify sequences flanking the transposon insertion (Parinov et al., 1999). The amplified product was sequenced. The site of insertion was confirmed by Southern analysis using as the probe a genomic fragment amplified using the primers Set3′ (5′-TCTCGGAGCAAGGTAATGGAG-3′) and R16 (5′-AAAGTTTGATCCAAAAACTGACTTTACAAA-3′) that were specific to At1g66170. To obtain genomic sequences flanking the Ds element, Ds-specific primers from both the 5′ and 3′ ends, Ds5-2 (5′-CGTTCCGTTTTCGTTTTTTACC3′) and Ds3-2 (5′-CCGGTATATCCCGTTTTCG-3′) were used in combination with gene-specific primers set5′ (5′-GTAACTCACGTTCACGCGTTA-3′) and Set3′ respectively. Double mutant analysis duet (Ler) as the female parent was crossed to dyad (Col) as the male parent. F1 and F2 were selected on MS plates containing kanamycin at 50 µg/ml. 96 F2 plants were transferred to soil; 49 of these were sterile. Plants homozygous for dyad were identified by examination of ovules as described previously (Agashe et al., 2002). The presence of the insertion and wild-type alleles at the duet locus was examined by PCR using a gene-specific primer R12 (5′-ATTCTCTGAACTTGGAAACTCATACTTTGG-3′) in combination with Ds5-2 for the insertion allele, and two gene-specific primers Dhf4 (5′-GTAGTAGATGGCCTGTGAGGAGACTAAT-3′) and STR5 (5′-TCTGC- Arabidopsis meiotic chromosome organization 5977 AAATTCTTCACAGCAATTCG-3′) on either side of the insertion site for the wild-type allele. DNA was isolated from one or two rosette leaves using the Nucleon Phytopure kit (Amersham). Pollen viability was measured using fluorescein diacetate according to the method of Heslop-Harrison and Heslop-Harrison (Heslop-Harrison and HeslopHarrison, 1970). To determine the effect of duet/duet on female fertility 10 plants that were homozygous for the duet allele and had normal ovules (dyad/+ or +/+) were crossed as the female parent to dyad as the male parent. All the siliques elongated and showed greater than 50% seed set after crossing, indicating no significant defect in female fertility. Fluorescence microscopy of meiotic chromosomes Analysis of meiotic chromosome spreads of male meiocytes was carried out according to the method of Ross et al. (Ross et al., 1996) with minor modifications (Agashe et al., 2002). Chromosomes were observed on a Zeiss Axioplan2 imaging microscope using a 365 nm excitation, 420 nm long-pass emission filter and a 100× oil objective. The photographs were captured on an Axiocam CCD camera (Carl Zeiss) using the Axiovision program (version 3.1) and were edited with Adobe Photoshop 5.0. Results was caused by a transposon insertion, the mutant was crossed as the female parent to a line that was homozygous for an insertion carrying the Ac transposase expressed under control of the 35S promoter (Sundaresan et al., 1995). The F1 plants were fertile and the segregation of the mutant phenotype in the F2 was consistent with it being a single gene recessive trait (Table 2). Out of a total of 51 sterile F2 plants, 17 were found to contain a revertant sector bearing one or more elongated siliques (Fig. 1C). The reversion phenotype was confirmed for nine independent sectors in the F3 generation by the presence of fertile plants among the progeny. Multiple plant progeny from seven of the nine sectors were tested by PCR for both the presence and absence of the Ds at the original site of insertion in At1g66170. Three out of the seven sectors lacked Ds indicating that excision had occurred from both chromosomes and was associated with the reversion to fertility. Genomic DNA flanking the site of excision was amplified by PCR and sequenced for multiple plants from each of the three sectors lacking Ds (Fig. 1F). Two out of the three sectors contained one wild-type and one mutant allele carrying the same 7 bp footprint at the excision site. The third sector contained one The duet mutant is male sterile because of a Ds transposon insertion The duet mutant was identified as a sterile line, SET8286, carrying a Ds enhancer trap insertion (Parinov et al., 1999). An examination of flowers showed that the mutant anthers lacked pollen. To determine the reason for sterility, reciprocal crosses were carried out to wild type. The results indicated that the mutant was male sterile and that female fertility was normal (Table 1). The anther filament did not fully elongate and remained below the level of the stigma (Fig. 1E), a feature that has also been observed in other male sterile lines (Sanders et al., 1999). Southern analysis indicated the presence of a single Ds insertion in the duet mutant (data not shown). DNA flanking the insertion site was isolated using TAIL PCR (Liu et al., 1995) and sequenced. The sequence indicated the insertion to be within the putative gene At1g66170. Southern analysis using a wild-type genomic clone as a probe confirmed the site of insertion (data not shown). To test whether the phenotype Table 1. The duet mutation causes male sterility Female parent Wild type duet Wild type Male parent Number of seeds per silique duet Wild type Wild type 0 25.8±2.8 27.2±1.8 Reciprocal crosses between duet and wild type, both in the Ler background were conducted to measure the seed yield. The results represent the mean and standard deviation from a minimum of eight crosses. Table 2. Segregation of the duet phenotype Wild type:mutant Observed Expected χ2 194:51 183.75:61.25 (3:1)* 228.66:16.33 (15:1)† 2.18, P>0.1 70.7, P<<0.001 *Mutant phenotype results from a single gene recessive mutation. †Two unlinked mutations are responsible for the mutant phenotype. Fig. 1. Wild-type (Ler), duet and revertant plants. (A) Wild-type plant showing normal elongating siliques. (B) duet mutant plant with short siliques. (C) Mutant plant with revertant sector showing elongating silique (arrow). (D) Wild-type flower with long anther filaments and plentiful pollen. (E) duet flower with short filaments and anthers lacking pollen. (F) Wild-type cDNA and derived amino acid sequence of DUET near the site of the Ds insertion. The duet mutant sequence shows a 8 bp duplication (bold underlined). The two excision alleles have a 7 and 8 bp footprint respectively (bold). 5978 Development 130 (24) Research article Fig. 2. Anther and pollen development in wild type and duet. Plastic cross-sections of anthers. (A,D,G) Wild type; (B,C,E,F,H,I) duet. (A,B) Anthers with pollen mother cells at stage 5, all the layers of the anther are present in both genotypes. (C) duet microspore mother cell at meiosis; pollen mother cells (PMCs) surrounded by a layer of callose. (D) Stage 7 anther showing tetrads held together by a layer of callose. (E) duet anther with dyads, triads, tetrads: products of a defective meiosis. (F) Products of aberrant meiosis separate out, enlarge and undergo nuclear division (arrowheads). (G) Stage 12 anther containing mature pollen. (H) Enlarged microspore-like cells (arrowheads) with a single nucleus and lacking exine. (I) Late stage anther showing empty locules. CL, callose layer; E, epidermis; En, endothecium; Ml, middle layer; PMC, pollen mother cell; T, tapetum; Tds, tetrads. Scale bars: 25 µm (A,B,C,F,G,I) and 12.5 µm (D,H,E). wild-type and two mutant alleles carrying the 7 bp and an 8 bp footprint, respectively. We found one plant that was heteroallelic for the two mutant alleles and was sterile. This is consistent with the development of the pollen that is carrying the mutant excision allele being associated with a multiple event that gave rise to the wild-type allele together with the mutant allele in the sporophyte. These data show that the duet mutant phenotype is due to the Ds transposon insertion. Microsporogenesis is defective in duet To examine the basis of male sterility and lack of pollen in the duet mutant we carried out a stagewise analysis of anther and pollen development by examining cleared anthers as well as plastic sections. Early stages of pollen development corresponding to anther stage 5 (Sanders et al., 1999) were normal (Fig. 2). The endothecium and internal layers surrounding the microsporocyte were indistinguishable from wild type as was the appearance of the microsporocyte prior to meiosis. We compared the time course of pollen development in wild type and duet by examination of cleared anthers and ovules from successive buds of inflorescences. Meiocyte division was prolonged (Table 3) and extended across three to four successive bud positions in the mutant inflorescence whereas in wild type it covered no more than two successive bud positions. The major product of division of the meiocyte was a pair of cells instead of a normal tetrad that is observed in wild type (Fig. 3). Tetrads were seen only rarely (Fig. 2E). The pair of cells separated and formed enlarged cells that were uninucleate (Fig. 2H, Fig. 3I). Subsequently nuclear division took place and cells were seen to contain one to three nuclei. Normal microspores were not observed and the exine was not formed. The enlarged cells later degenerated, leaving an empty locule (Fig. 2I). Mutant meiocytes at the time of division had a prominent callose wall (Fig. 2C) Table 3. Pollen development in wild type and duet relative to ovule stages Ovule stage 1-2 2-1 2-2 2-3 2-4 2-5 3-1 3-2 Wild type Microsporocytes Tetrads Free microspores (expanding) Microspores with exine Vacuolated microspores Mature pollen duet Microsporocytes Separated meiocytes Dyads Dyads (separating) Uninucleate spores Uni, binucleate spores Multinucleate spores Spores degenerate Three inflorescences each for wild type and duet were analyzed. Ovule stages are according to Schneitz et al., (Schneitz et al., 1995). Arabidopsis meiotic chromosome organization 5979 Fig. 3. Stages of male meiosis and pollen development in wild type and duet. Optical sections of cleared anthers viewed under DIC optics. (A-D) Wild type; (E-K) duet. (A,E) Stage 5 anthers with pollen mother cells (arrowheads). (B) Stage 7 anther containing tetrads (arrowhead). (C) Microspores released from the tetrad generate an exine wall and become vacuolated (arrowhead). (D) Mature pollen. (F,G) Dyads (arrowhead in F) formed after a defective meiosis. (H,I) Cells of the dyad separate and enlarge (arrowhead in H). (J,K) The microspore-like cells released from the dyad enlarge and undergo nuclear division to form 2-3 nucleate cells (arrowhead in J), which later degenerate. Scale bars: 12.5 µm. whereas the pair of cells formed by division of the meiocyte lacked a thick callose wall and separated into single cells. This probably corresponds to loss of the callose wall from tetrads at the time of microspore release in wild type. The stage at which the pair of cells separated in the duet mutant corresponded to ovule stage 2-4 which is later than the time of microspore release in wild type (ovule stage 2-2; Table 3). Taken together these data indicate that duet is defective in male meiotic progression. DUET potentially encodes a PHD finger protein Based on the predicted cDNA sequence of At1g66170, PCR primers were designed to span the coding region. RT-PCR was carried out using cDNA prepared from inflorescence mRNA. The sequence of the cDNA obtained (GenBank accession no. AY305007) indicated the presence of 3 exons potentially encoding a protein of 704 amino acids and having a mass of 80.8 kDa (Fig. 4). The Ds insertion is in the third exon at a position corresponding to aa 550 and hence likely to create a null allele. The putative protein was similar to that of the AGI annotation but contained an additional exon that was not present in the annotated sequence. A potential nuclear localization signal was found at amino acid residues 10-15. A homology search using BLASTP 2.2.6 revealed the presence of a PHD finger domain towards the C-terminal portion of the predicted DUET protein, from aa 609-656. The PHD domain is a modified zinc finger thought to be involved in transcriptional regulation and chromatin organization (Aasland et al., 1995). The closest known protein to DUET was the MALE STERILITY 1 (MS1) protein (expectation value, E=4×10–83), which has been proposed to be a transcriptional regulator of male gametogenesis and also contains a C-terminal PHD finger domain (Wilson et al., 2001). In addition, two predicted genes from Arabidopsis (At1g33420, and At2g01810) and one from rice (GenBank ID AC090882) showed similarity to DUET. All three predicted proteins have a PHD domain towards the C-terminal end. All five genes show similarity throughout their length and hence constitute a gene family. Apart from the above genes, which showed strong similarity to DUET, weak similarity was detected to other Arabidopsis genes including SWI1/DYAD (E=1×10–4). The SWI1/DYAD gene has been shown to be required for chromosome cohesion in meiosis and for female meiotic progression (Mercier et al., 2001; Agashe et al., 2002). The SWI1/DYAD homology resides towards the middle portion of the gene from aa 309 to 395. DUET is expressed in male meiocytes Expression of the DUET gene was examined using RT-PCR. The presence of the transcript could be detected in the 5980 Development 130 (24) Research article Fig. 4. (A) Schematic representation of the DUET gene. The exons are indicated with black boxes. Arrows indicate the primers used for cDNA isolation and expression analysis. Coordinates are with respect to BAC F15E12. (B) The predicted sequence of DUET protein. The putative nuclear localization signal is in bold. The region showing homology to DYAD is underlined and the PHD-finger domain is boxed. The inverted triangle after amino acid 550 indicates the Ds transposon insertion site. (C) Analysis of DUET expression by RT-PCR. Expression of the DUET gene was examined in wild type (Wt) leaves (Lea), inflorescence (Inf) and duet mutant inflorescence by amplifying the cDNA synthesized from poly(A)+ mRNA. The shift in the size of the amplicon can be observed when genomic DNA (gen) was used as template. The constitutive GAPC gene was used as the normalization control. (D) Comparison of DUET and DYAD cDNA in pistils. inflorescence but not in leaves (Fig. 4C). The mutant did not show expression under these conditions and hence is likely to be a null allele. We have previously demonstrated the presence of DYAD mRNA specifically in male and female meiocytes (Agashe et al., 2001). To test whether DUET expression is male specific, we compared the levels of DUET message with that of DYAD in dissected pistils using RT-PCR. We did not observe expression of DUET whereas DYAD expression could be detected under the same conditions (Fig. 4D). To determine the DUET expression pattern in the inflorescence at the cellular level we carried out RNA in-situ hybridization using antisense RNA complementary to DUET cDNA excluding the PHD domain as a probe. Expression was first observed in sporogenous cells at late anther stage 4 (Sanders et al., 1999) (Fig. 5A), reached a maximum in male meiocytes at anther stage 5, prior to meiosis (Fig. 5C). Lower expression was observed at anther stage 6, during meiosis (Fig. 5D) and subsequently declined. A weak signal could be seen in very young pistils in the placenta, corresponding to the presumptive site of ovule initiation (Fig. 5B,C). We did not see expression in female meiocytes or in ovules (Fig. 5E,F). The lack of female meiocyte expression as well as a phenotype in what appears to be a null allele, would suggest that DUET does not have a function in the female meiocyte. We also examined GUS reporter gene expression in plants hemizygous and homozygous for the insertion but did not observe expression in anthers at stages 5 to 6. Aberrant meiotic chromosome organization and metaphase 1 arrest in the duet mutant Meiotic chromosome stages in wild type and duet were analyzed in spread chromosome preparations from anthers of Fig. 5. Expression of DUET in male meiocytes: RNA in situ hybridization of DUET antisense RNA to sections of flower buds. (A) Expression is first seen in sporogenous cells at anther stage 4. (B,C) Maximal expression is seen in microsporocytes at anther stage 5. (D) Anther stage 6, meiotic cells. (E,F) No expression is seen in the female meiocyte at stage 2-3 corresponding to pre-meiosis. (E) Antisense; (F) sense control. Scale bars: 50 µm. Arabidopsis meiotic chromosome organization 5981 meiotic stage buds using the method of Ross et al. (Ross et al., 1996). Chromosome development in duet appeared normal through early stages of meiotic prophase up to pachytene. Abnormalities first became noticeable at diplotene when duet chromosomes started to appear somewhat diffuse in comparison to wild type (Fig. 6D,N). During diplotene and diakinesis, duet chromosomes were observed to desynapse along much of their length including centromeres, to a greater extent than was observed for wild type (Fig. 6N-P). At diakinesis, duet chromosomes were irregular and less distinct than wild type (Fig. 6P,Q). Metaphase 1 in duet varied from nearly normal (Fig. 6R) to a diffuse mass where individual chromosomes could not be clearly distinguished (Fig. 6T). In wild type, male meiotic stages extended across no more than two successive bud positions in an inflorescence whereas in duet, meiotic stages covered three to five bud positions. The total number of meiotic stages in an inflorescence was also several times greater for duet than for wild type. This could be approximately gauged from the fact that 364 meiotic stages were counted from six inflorescences in the case of wild type and 602 from three Fig. 6. Chromosome analysis in spreads of male meiocytes in wild type and duet. (A-J) Wild type; (K-W) duet. (A,K) Chromosomes first become visible as elongated strands during leptotene. (B,L) Synapsis takes place during zygotene. (C,M) Synapsis is complete at pachytene and chromosomes have a shorter and thicker appearance. (D) Diplotene stage, when bivalents have undergone partial decondensation. (E) Late diakinesis showing five pairs of chromosomes with chiasmata at their ends. (F) Metaphase I stage showing five condensed bivalents arranged on a metaphase plate. (G) Telophase I: five chromosomes at each end are separated by an organelle band. (H) Metaphase II. (I) Anaphase II. (G-I) Arrows indicate the densely compacted organelle band. (J) Telophase II where four groups of five chromosomes each have separated. (N) First apparent visible defect in duet at diplotene. Chromosomes start to look diffuse and two bivalents have undergone partial desynapsis (arrowheads). (O) A more severe form of desynapsis can be observed in the majority of bivalents. (P,Q) Disorganized diakinesis in duet with diffuse chromosomes including the centromeric region. (R,S,T) Metaphase I. (U) Anaphase I. (V) Defective anaphase I stage with fragmented chromosome and laggards. (W) Telophase I. The organelle band is absent. Scale bars: 12.5 µm. 5982 Development 130 (24) The duet dyad double mutant flowers lacked viable pollen. Stagewise analysis of pollen development in cleared anthers (Fig. 8) showed meiocytes followed by enlarged uninucleate cells that underwent further enlargement to produce binucleate cells. We did not observe clear cytological evidence of meiotic divisions. At a later stage anthers contained irregular enlarged cells containing multiple nuclei and surrounded by an irregular cell wall that resembled the exine. The exine-like structure was not observed in the duet single mutant. Buds from plants that were duet/+ dyad/dyad showed reduced numbers of pollen grains compared to the corresponding single mutants. Anthers from freshly opened flowers were dissected and tested for pollen viability. The mean pollen viability for duet/+ dyad/dyad was 34±12% whereas for plants that were duet/+ +/– the pollen viability was 75±13%. The total number of The duet mutation interacts with dyad pollen grains was also lower for the interaction genotype. A The duet mutant phenotype is similar to what is seen for female rough indication of this could be obtained from the total meiosis in the dyad mutant (Siddiqi et al., 2000). In each case amount of pollen on the slide. For the duet/+ dyad/dyad a pair of cells is formed after meiosis instead of a tetrad. The genotype the mean count of total pollen grains per flower was pair of cells do not develop further into functional gametes. For 131±50 whereas for sibling plants that were duet/+ +/– the both duet and dyad, the two-cell phenotype correlates with mean pollen count was 326±171. Each individual genotype defective progression through meiosis and the underlying duet/+ and dyad/dyad produced numbers of viable pollen that cause appears to be altered chromosome organization in both were comparable to wild type (Siddiqi et al., 2000; data not cases. The DYAD/SWI1 gene is required for female meiotic shown). Analysis of pollen development in cleared anthers progression, and for chromosome cohesion in male and female showed that male meiocytes in duet/+ dyad/dyad plants meiosis (Mercier et al., 2001; Agashe et al., 2002). However, underwent an aberrant division to produce mostly dyads and the dyad mutant allele is female specific and shows normal triads, as well as some tetrads (Fig. 8). The majority of spores pollen development and male fertility. produced were defective and formed enlarged cells containing To test if both mutants are affected in a related aspect of 1-3 nuclei and that subsequently degenerated. The phenotype chromosome organization during meiosis, we intercrossed duet therefore broadly resembled that of homozygous duet plants and dyad as female and male parents respectively to test for though it was less severe. genetic interaction. The F1 were fully fertile and showed Examination of meiotic chromosome behavior revealed normal pollen and embryo sac development (data not shown). differences in duet/+ dyad/dyad plants from that observed in F2 plants were genotyped with respect to the alleles present at homozygous duet plants. The early stages of meiotic prophase the duet locus (duet/duet, duet/+, and +/+) and at the dyad were detected though aberrant chromosome morphology was locus (dyad/dyad, and +/–), and various dose combinations of apparent at the pachytene stage (Fig. 9B). Diakinesis was duet and dyad were examined for evidence of genetic variable with some being nearly normal and having all or a interaction. majority of the chromosomes retaining their bivalent structure (Fig. 9C). In others, many of the chromosomes dissociated into univalents (Fig. 9D). A more 60 extreme phenotype observed at diakinesis was the loss of sister chromatid cohesion resulting in 50 dissociation of chromosomes into isolated chromatids (Fig. 9E). The ensuing meiosis 1 40 divisions spanned the range from a normal reductional division (these were relatively rare and not observed, but their existence could be 30 inferred from the production of tetrads and viable pollen) to an equational mitotic-like division in 20 which univalents separated into sister chromatids (Fig. 9G,H), to an unequal division arising from 10 random segregation of univalents and single chromatids (Fig. 9I,J). We did not observe a high 0 proportion of metaphase 1 arrest which L Z P DP DI M -I A-I T-I M-II A-II T-II TD correlated with a reduction in the number of cell Meiotic Chromosome Stages pairs that were seen compared to the duet single mutant. The homozygous double mutant Fig. 7. Quantitation of different stages of male meiosis in wild type and duet. combination was also examined. Here too (White bars) Wild type; (Black bars) duet; leptotene (L), zygotene (Z), pachytene bivalents were observed to separate into (P), diplotene (DP), diakinesis (DI), metaphase I (M-I), anaphase I (A-I), telophase univalents at diakinesis (Fig. 9L) and in some I (T-I), metaphase II (M II), anaphase II (A-II), telophase (T-II) and tetrads (TD). Note the large increase in the proportion of metaphase I cells in duet. meiocytes chromosome cohesion was lost Percent total inflorescences for duet. In most of the buds there was an excess of metaphase 1 stages in the duet mutant (Fig. 7), whereas these were relatively few in wild type. The fraction of anaphase 1 meiocytes was also greater for duet. A clear feature of duet meiocytes was the absence of a band of organelles characteristic of wild type and prominently visible starting at telophase 1 in the central portion of the meiocyte (Fig. 6G). Instead organelles were dispersed throughout the cytoplasmic region of the male meiocyte in the case of duet (Fig. 6W). These data confirm and extend the analysis of male meiocyte division in the duet mutant. The results indicate that duet has a defect in chromosome organization during male meiosis and is also defective in male meiotic progression. Many of the meiocytes arrest at metaphase 1. Research article Arabidopsis meiotic chromosome organization 5983 Fig. 8. Interactions between duet and dyad during male meiosis and pollen development. Optical sections of cleared anthers viewed under DIC optics. (A-D) duet dyad double mutant; (E-L) duet/+ dyad/dyad. (A,E) Normal-looking meiocytes (arrowheads). (B) Defective microspores lacking exine (arrowhead). (C) Binucleate spores. (D) Late stage defective binucleate and multinucleate spores (arrow and arrowhead, respectively) surrounded by an uneven wall resembling the exine; not observed in the duet single mutant. (F) Dyad (arrowhead). (G) Undivided triads (arrowhead). (H) Round spores containing 1-3 nuclei. (I) Normal-looking tetrads (arrowhead). (J) Normal-looking developing pollen. (K) Abnormal multinucleate structures undergoing shrinkage (arrowhead). (L) Shrunken pollen. Scale bar: 12.5 µm. resulting in isolated chromatids (Fig. 9M). At prometaphase between eight and ten thick and diffuse chromosomes could generally be counted, most probably they were a mixture of univalents and bivalents (Fig. 9N). Male meiotic chromosomes in the dyad mutant showed no apparent differences from wild type. In the case of duet/+ also, the vast majority of meiotic structures observed were normal, in agreement with the essentially wild-type levels of viable pollen that were measured in freshly opened flowers. However, in one inflorescence out of four examined we observed a single bud in which a minority (<10%) of meioses were aberrant. Chromosomes in this bud showed defects in diakinesis that were similar to those observed in homozygous duet, and some meiocytes were arrested at metaphase 1 (data not shown). Separated microspores were the major species present in this bud indicating that the majority of meiocytes had completed meiosis, and that the minority, showing defective meiotic structures, were defective in progression. The increased severity of defects in pollen development resulting from defects in male meiosis that were observed in duet/+ dyad/dyad plants, coupled with the loss of chromosome cohesion provide evidence of a synergistic interaction between the duet and dyad mutant alleles. To test for possible effects of duet on female meiosis we examined cleared ovules as well as female meiosis in duet dyad double mutant plants (Fig. 9U-W). The ovule phenotype was identical to that of the dyad mutant. In both cases the majority of ovules showed a single division meiosis and the presence of two enlarged cells in place of an embryo sac. Cytogenetic analysis of female meiosis in duet dyad plants also indicated that chromosome behavior was the same as that described earlier for dyad, which was shown to undergo an equational meiosis 1 division (Agashe et al., 2002). No additional effects were observed from the presence of the duet mutant allele in a dyad background, on the integrity or appearance of chromosomes. We also examined female fertility of duet/duet 5984 Development 130 (24) Research article Fig. 9. Interactions between duet and dyad: meiotic chromosome stages. (A-J) duet/+ dyad/dyad. (K-N,W) duet dyad double mutant. (O-Q) duet/+ heterozygote. (R-T,V) dyad mutant. (A) Early zygotene. (B) Pachytene stage showing thickened but irregular chromosomes. (C) Diakinesis. Five bivalents are visible. (D) Aberrant diakinesis in which chromosomes have desynapsed to form ten univalents. (E) Extreme diakinesis in which both synapsis and sister chromatid cohesion have been lost to yield single chromatids. (F) Early anaphase 1 undergoing mixed segregation in which both univalents and bivalents are involved. (G) Late anaphase 1 showing approximately equal separation of chromosomes. Eight to ten chromosomes are present at each pole indicating an equal division. (H) Telophase 1. Equal division. Ten chromosomes are present at each pole. (I) Telophase 1. Unequal division. (J) Dyad formed after unequal division. (K) Zygotene. (L) Diakinesis involving 2 bivalents and 6 univalents. (M) Extreme diakinesis containing mostly single chromatids. (N) Prometaphase 1 having eight to ten thick diffuse chromosomes. (O,R) Normal diakinesis. (P,S) Metaphase 1. (Q,T) Telophase 1. (U,V) Cleared ovules of dyad (U) and duet dyad (V). (W) Metaphase 1. Scale bars: 12.5 µm (A-T,W) and 25 µm (U,V). dyad/+ plants and did not observe any reduction in fertility (data not shown). We therefore did not find any evidence to support a role for DUET in female meiosis. Discussion Progression through the meiotic cell cycle requires orchestration of a set of events that includes the assembly of chromosomes for reductional division, pairing of homologous chromosomes, recombination and segregation of homologues to opposite poles of the meiosis 1 spindle (reviewed by Dawe, 1998). Defects in meiotic chromosome organization can result in delayed progression through the meiotic cell cycle. The role of chromosomal checkpoints in monitoring and ordering the phases of the mitotic and meiotic cell cycle is well documented in yeast and animals (Roeder and Bailis, 2000; Handel et al., 1999). In plants there is little information on the control of meiotic progression and the role of chromosomal checkpoints in meiosis (Garcia et al., 2003). Mutations in the yeast DMC1 gene cause arrest as a result of activation of the pachytene Arabidopsis meiotic chromosome organization 5985 checkpoint (Bishop et al., 1992; Lydall et al., 1996). However, a mutation in AtDMC1, the Arabidopsis homolog of DMC1 does not cause arrest. Instead it leads to random chromosome segregation in meiosis and the production of defective spores (Couteau et al., 1999). Hence the control of meiotic progression in plants may differ from that in yeast. The properties of the DUET gene described above indicate that it is required for male meiotic chromosome organization and suggest that in the absence of DUET function, male meiocytes undergo defective progression through the meiotic cell cycle. In the duet mutant, male meiocytes went through a single division to produce a pair of cells instead of a normal tetrad. The defective spores produced did not complete gametogenesis and degenerated. The phenotype resembled that observed in the dyad mutant of Arabidopsis in which the majority of female meiocytes divide singly to give a dyad followed by an arrest in further development of the female gametophyte (Siddiqi et al., 2000). Single division meiosis has also been described for the spo12, spo13 and slk19 mutants of yeast (Klapholz and Esposito, 1980; Zeng and Saunders, 2000; Kamieniecki et al., 2000). The DUET gene was cloned by transposon tagging with a Ds element and found to encode a putative PHD finger domain protein. The gene is closely related to the MS1 gene, which has been proposed to be a transcriptional regulator of male gametogenesis in Arabidopsis (Wilson et al., 2001), to two other putative Arabidopsis genes, At1g33420 and At2g01810, and to a putative rice gene (GenBank ID AC090882). The PHD finger is a modified zinc finger and is found in a number of proteins that play a role in chromatin organization and transcriptional regulation and include members of the Trithorax and Polycomb groups (reviewed by Aasland et al., 1995). In plants the PHD finger has been found in a transcriptional regulator of genes involved in defense against pathogens (Korfhage et al., 1994) and in genes that are required for reproductive development and fertility: the PICKLE gene is required to prevent re-expression of embryonic traits in germinated seedlings and encodes a CHD3 domain protein that has been proposed to act as a regulator to promote the transition from embryonic to postembryonic development (Ogas et al., 1999); overexpression of the SHL gene has been show to lead to early flowering and defective reproductive development whereas antisense inhibition caused dwarfism and delayed growth (Mussig et al., 2000). Hence, in both plants and animals PHD finger genes play a role in developmental transitions. Recently the PHD finger domain has also been found in proteins that act as E3 ubiquitin ligases (Cosoy and Ganem, 2003). This latter class of PHD finger proteins are localized to the membrane or cytoplasm. The PHD finger in DUET is more similar to that found in proteins that act as chromatin remodeling factors or transcriptional regulators. The DUET gene also showed limited similarity to SWI1/DYAD a gene that has been demonstrated to be required for chromosome cohesion during meiosis in Arabidopsis (Mercier et al., 2001; Agashe et al., 2002). Expression of the DUET gene in the inflorescence appeared to be specific to the male meiocyte. Earliest expression was detected in stage 4 anthers at a time that corresponds to the presence of sporogenous cells. Maximal expression was seen at anther stage 5 prior to meiosis, after which the signal declined. Since the expression and phenotype for DUET and the related MS1 gene appears to be sex specific (Wilson et al., 2001; Ito and Shinozaki, 2002), it is possible that along with the other two closely related putative genes At1g33420 and At2g01810, they define a family of transcriptional regulators that function during male meiosis and gametogenesis. The appearance of chromosomes during early stages of meiotic prophase in the duet mutant was normal up to pachytene. Differences from wild type first became noticeable at diplotene with chromosomes appearing more irregular and diffuse in the mutant. This would suggest that either the timing of DUET action is at the onset of diplotene or else that DUET may act earlier, but its absence may lead to visible changes in chromosome structure only at a later stage when the synaptonemal complex is disassembled and most of the sister chromatid cohesion is removed at diplotene. The difference between duet and wild type was accentuated at diakinesis and culminated in a high proportion of meiocytes showing arrest at metaphase 1. The appearance of chromosomes at metaphase 1 was variable and distinctly different from wild type. The metaphase 1 phenotype ranged from nearly normal looking structures to ones in which the chromosomes appeared as an irregular mass towards the center of the cell in which individual chromosomes could not be clearly distinguished. A distinct characteristic of the mutant meioses was the absence of the organelle band which is a prominent feature found at the center of the cell of wild-type meiocytes at telophase 1. Instead, the organelles in the mutant meiocytes appeared more evenly distributed throughout the cytoplasm. The reason for this could be that the localization of mitochondria and plastids in the dividing meiocyte requires expression of specific genes during meiosis, and that the expression of these genes is adversely affected in the mutant. Alternatively the cause could be a more general disruption of cytoplasmic and cytoskeletal organization that affects organelle transport and localization in the meiocyte. The finding of defects in chromosome organization in meiosis caused by disruption of the DUET gene extends the role of PHD finger proteins to include functions that are specific to meiosis. Double mutant combinations of duet with dyad revealed genetic interaction manifesting in defects during male meiosis. The effect was most apparent in plants that were duet/+ dyad/dyad. Whereas the individual dyad/dyad and duet/+ plants showed no or very weak effects on pollen development, the combination resulted in strong defects in male meiosis. Microsporocytes showed defective division patterns and the products were dyads, triads and tetrads. Most of the microspores produced were defective and degenerated. However a minority of spores did develop into viable pollen. At the cellular level, the defective divisions of the male meiocyte were similar to those in duet/duet, although less severe. The duet dyad double mutant showed a progression defect that was more severe than in the duet single mutant as the male meiocytes failed to divide. At the chromosomal level there were additional defects that were not observed in either single mutant. In duet/+ dyad/dyad meiocytes, chromosomes lost synapsis or cohesion prior to meiosis 1 and segregated unequally in many cases. Loss of cohesion was not observed in either the dyad or duet single mutants. dyad is a femalespecific allele and shows normal male meiosis and pollen development (Siddiqi et al., 2000). The stronger allele swi1-2 is male sterile and shows loss of sister chromatid cohesion 5986 Development 130 (24) during prophase of male meiosis (Mercier et al., 2001). Hence the loss in sister chromatid cohesion as a result of the interaction may be interpreted to mean that hemizygous duet/+ enhances the dyad mutant phenotype. The genetic interaction between duet and dyad could be specific and the genes may act at the same level of chromosome architecture. The presence of the PHD finger in DUET implicates it as functioning in the control of transcription at the level of chromatin organization whereas DYAD/SWI1 has been shown to function in chromosome organization and cohesion. If DUET does in fact function as a transcriptional regulator, this would point to a close connection between cohesion and the control of transcription at the chromatin level during meiosis. The formation of dyads and the fact that meiosis is more extended in the duet mutant clearly suggests a defect in meiotic progression. Analysis of single division meiosis in the spo13 mutant of yeast has shown that the basis for the progression defect is the activation of the spindle checkpoint. In the absence of the spindle checkpoint, spo13 mutants undergo normal meiotic progression and form four spores (Shonn et al., 2002; Lee et al., 2002). There is at present limited information on the control of meiotic progression in plants. Immunolocalization of a maize homologue of the yeast spindle checkpoint protein MAD2 has shown that it is expressed in meiosis and localized to the kinetochore where it functions through a tensiondependent mechanism (Yu et al., 1999). Hence the basic apparatus for the spindle checkpoint is conserved in plants. A recent study has identified a mutant sog1 that suppresses gamma radiation-induced arrest and also affects pollen development (Preuss and Britt, 2003). The further analysis of mutants defective in meiotic progression should provide information on the existence of chromosomal checkpoints in plant meiosis. This work was supported by the Council for Scientific and Industrial Research, Government of India. T.V.R., B.A. and J.K. are recipients of CSIR Research Fellowships. This work was also partly supported by grants from NSTB Singapore to V.S. We thank Dr Shashi Singh for help with sectioning of plant material and Mehar Sultana for synthesis of oligonucleotides. We also acknowledge the ABRC for DNA clones and seed material. Note added in proof While the manuscript was under review, Yang et al. reported the cloning and expression analysis of the MMD gene, and analysis of the mmd mutant. The MMD gene is identical to DUET (Yang, X. et al., 2003). References Aasland, R., Gibson, T. J. and Stewart, A. F. (1995). The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 20, 56-59. Agashe, B., Prasad, C. K. and Siddiqi, I. (2002). Identification and analysis of DYAD: a gene required for meiotic chromosome organisation and female meiotic progression in Arabidopsis. Development 129, 3935-3943. Armstrong, S. J., Caryl, A. P., Jones, G. H. and Franklin, F. C. (2002). Asy1, a protein required for meiotic chromosome synapsis, localizes to axisassociated chromatin in Arabidopsis and Brassica. J. Cell. Sci. 15, 36453655. Azumi, Y., Liu, D., Zhao, D., Li, W., Wang, G., Hu, Y. and Ma, H. (2002). Homolog interaction during meiotic prophase I in Arabidopsis requires the SOLO DANCERS gene encoding a novel cyclin-like protein. EMBO J. 21, 3081-3095. Research article Bai, X., Peirson, B. N., Dong, F., Xue, C. and Makaroff, C. A. (1999). Isolation and characterization of SYN1, a RAD21-like gene essential for meiosis in Arabidopsis. Plant Cell 11, 417-430. Bhatt, A. M., Canales, C. and Dickinson, H. G. (2001). Plant meiosis: the means to 1N. Trends Plant Sci. 6, 114-121. Bishop D. K., Park, D., Xu, L. and Kleckner, N. (1992). DMC1: a meiosisspecific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69, 439456. Byzova, M. V., Franken, J., Aarts, M. G., de Almeida-Engler, J., Engler, G., Mariani, C., Van Lookeren Campagne, M. M. and Angenent, G. C. (1999). Arabidopsis STERILE APETALA, a multifunctional gene regulating inflorescence, flower, and ovule development. Genes Dev. 13, 1002-1014. Canales, C., Bhatt, A. M., Scott, R. and Dickinson, H. (2002). EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr. Biol. 12, 1718-1727. Chaudhury, A. M., Lavithis, M., Taylor, P. E., Craig, S., Singh, M. B., Singer, E. R., Knox, R. B. and Dennis, E. S. (1994). Genetic control of male fertility in Arabidopsis thaliana: Structural analysis of premeiotic developmental mutants. Sex. Plant Reprod. 7, 17-28. Chen, C., Marcus, A., Li, W., Hu, Y., Calzada, J. P., Grossniklaus, U., Cyr, R. J. and Ma, H. (2002). The Arabidopsis ATK1 gene is required for spindle morphogenesis in male meiosis. Development 129, 2401-2409. Cosoy, L. and Ganem, D. (2003). PHD domain and E3 ubiquitin ligases: Viruses make the connection. Trends Cell Biol. 13, 7-12. Couteau, F., Belzile, F., Horlow, C., Grandjean, O., Vezon, D. and Doutriaux, M. P. (1999). Random chromosome segregation without meiotic arrest in both male and female meiocytes of a dmc1 mutant of Arabidopsis. Plant Cell 11, 1623-1634. Curtis, C. A. and Doyle, G. G. (1991). Double meiotic mutants of maize: implications for the genetic regulation of meiosis. J. Hered. 82, 156-163. Dawe, R. K. (1998). Meiotic chromosome organization and segregation in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 371-395. Dawe, R. K. and Freeling, M. (1990). Clonal analysis of the cell lineages in the male flower of maize. Dev. Biol. 142, 233-245. Edelmann, W., Cohen, P. E., Kane, M., Lau, K., Morrow, B., Bennett, S., Umar, A., Kunkel, T., Cattoretti, G., Chaganti, R. et al. (1996). Meiotic pachytene arrest in MLH1-deficient mice. Cell 85, 1125-1134. Garcia, V., Bruchet, H., Camescasse, D., Granier, F., Bouchez, D. and Tissier, A. (2003). AtATM is essential for meiosis and the somatic response to DNA damage in plants. Plant Cell 15, 119-132. Golubovskaya, I., Avalkina, N. and Sheridan, W. F. (1997). New insights into the role of the maize ameiotic1 locus. Genetics 147, 1339-1350. Grelon, M., Vezon, D., Gendrot, G. and Pelletier, G. (2001). AtSPO11-1 is necessary for efficient meiotic recombination in plants. EMBO J. 20, 589600. Handel, M. A., Cobb, J. and Eaker, S. (1999). What are the spermatocyte’s requirements for successful meiotic division? J. Exp. Zool. 285, 243-250. Heslop-Harrison, J. and Heslop-Harrison, Y. (1970). Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 45, 115-120. Huang, B. Q. and Sheridan, W. F. (1996). Embryo sac development in the maize indeterminate gametophyte1 mutant: Abnormal nuclear behavior and defective microtubule organization. Plant Cell 8, 1391-1407. Ito, T. and Shinozaki, K. (2002). The MALE STERILITY1 gene of Arabidopsis, encoding a nuclear protein with a PHD-finger motif, is expressed in tapetal cells and is required for pollen maturation. Plant Cell Physiol. 43, 1285-1292. Kamieniecki, R. J., Shanks, R. M. and Dawson, D. S. (2000). Slk19p is necessary to prevent separation of sister chromatids in meiosis I. Curr. Biol. 10, 1182-1190. Klapholz, S. and Esposito, R. E. (1980). Recombination and chromosome segregation during the single division meiosis in SPO12-1 and SPO13-1 diploids. Genetics 96, 589-611. Klimyuk, V. I. and Jones, J. D. (1997). AtDMC1, the Arabidopsis homologue of the yeast DMC1 gene: characterization, transposon-induced allelic variation and meiosis-associated expression. Plant J. 11, 1-14. Korfhage, U., Trezzini, G. F., Meier, I., Hahlbrock, K. and Somssich, I. E. (1994). Plant homeodomain protein involved in transcriptional regulation of a pathogen defense-related gene. Plant Cell 6, 695-708. Lee, B. and Amon, A. (2001). Meiosis: how to create a specialized cell cycle. Curr Opin Cell Biol 13, 770-777. Arabidopsis meiotic chromosome organization 5987 Lee, B. H., Amon, A. and Prinz, S. (2002). Spo13 regulates cohesin cleavage. Genes Dev. 16, 1672-1681. Liu, Y. G., Mitsukawa, N., Oosumi, T. and Whittier, R. F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8, 457-463. Lydall, D., Nikolsky, Y., Bishop, D. K. and Weinert, T. (1996). A meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature 383, 840-843. Magnard, J. L., Yang, M., Chen, Y. C., Leary, M. and McCormick, S. (2001). The Arabidopsis gene tardy asynchronous meiosis is required for the normal pace and synchrony of cell division during male meiosis. Plant Physiol. 127, 1157-1166. McCormick, S. (1993). Male gametophyte development. Plant Cell 5, 12651275. Mercier, R., Vezon, D., Bullier, E., Motamayor, J. C., Sellier, A., Lefevre, F., Pelletier, G. and Horlow, C. (2001). SWITCH1 (SWI1): a novel protein required for the establishment of sister chromatid cohesion and for bivalent formation at meiosis. Genes Dev. 15, 1859-1871. Mercier, R., Armstrong, S. J., Horlow, C., Jackson, N. P., Makaroff, C. A., Vezon, D., Pelletier, G., Jones, G. H., and Franklin, F. C. H. (2003). The meiotic protein SWI1 is required for axial element formation and recombination initiation in Arabidopsis. Development, 130, 3309-3318. Mussig, C., Kauschmann, A., Clouse, S. D. and Altmann, T. (2000). The Arabidopsis PHD-finger protein SHL is required for proper development and fertility. Mol. Gen. Genet. 264, 363-370. Ogas, J., Kaufmann, S., Henderson, J. and Somerville, C. (1999). PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc. Natl. Acad. Sci. USA 96, 13839-13844. Parinov, S., Sevugan, M., Ye, D., Yang, W. C., Kumaran, M. and Sundaresan, V. (1999). Analysis of flanking sequences from dissociation insertion lines: a database for reverse genetics in Arabidopsis. Plant Cell 11, 2263-2270. Preuss, S. B. and Britt, A. B. (2003). A DNA-damage-induced cell cycle checkpoint in Arabidopsis. Genetics 164, 323-334. Roeder, G. S. and Bailis, J. M. (2000). The pachytene checkpoint. Trends Genet. 16, 395-403. Ross, K. J., Fransz, P. and Jones, G. H. (1996). A light microscopic atlas of meiosis in Arabidopsis thaliana. Chromosome Res. 4, 507-516. Ross, K. J., Fransz, P., Armstrong, S. J., Vizir, I., Mulligan, B., Franklin, F. C. and Jones, G. H. (1997). Cytological characterization of four meiotic mutants of Arabidopsis isolated from T-DNA-transformed lines. Chromosome Res. 5, 551-559. Sanders, P. M., Bui, A. Q., Weterings, K., McIntire, K. N., Hsu, Y. C., Lee, Y. R., Troung, M. T., Beals, T. P. and Goldberg, R. B. (1999). Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 11, 2997-2322. Schiefthaler, U., Balasubramanian, S., Sieber, P., Chevalier, D., Wisman, E. and Schneitz, K. (1999). Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 96, 1166411669. Sheridan, W. F., Avalkina, N. A., Shamrov, I. I., Batygina, T. B. and Golubovskaya, I. N. (1996). The mac1 gene: controlling the commitment to the meiotic pathway in maize. Genetics. 142, 1009-1020. Shonn, M. A., McCarroll, R. and Murray, A. W. (2002). Spo13 protects meiotic cohesin at centromeres in meiosis I. Genes Dev. 16, 1659-1671. Siddiqi, I., Ganesh, G., Grossniklaus, U. and Subbiah, V. (2000). The dyad gene is required for progression through female meiosis in Arabidopsis. Development 127, 197-207. Sundaresan, V., Springer, P., Volpe, T., Haward, S., Jones, J. D., Dean, C., Ma, H. and Martienssen, R. (1995). Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 9, 1797-1810. Taylor, P. E., Glover, J. A., Lavithis, M., Craig, S., Singh, M. B., Knox, R. B., Dennis, E. S. and Chaudhury, A. M. (1998). Genetic control of male fertility in Arabidopsis thaliana: structural analyses of postmeiotic developmental mutants. Planta 205, 492-505. Wilson, Z. A., Morroll, S. M., Dawson, J., Swarup, R. and Tighe, P. J. (2001). The Arabidopsis MALE STERILITY1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD-finger family of transcription factors. Plant J. 28, 27-39. Yang, M., Hu, Y., Lodhi, M., McCombie, W. R. and Ma, H. (1999a). The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proc. Natl. Acad. Sci. USA 96, 1141611421. Yang, W. C., Ye, D., Xu, J. and Sundaresan, V. (1999b). The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 13, 21082117. Yang, W. C. and Sundaresan, V. (2000). Genetics of gametophyte biogenesis in Arabidopsis. Curr. Opin. Plant Biol. 3, 53-57. Yang, X., Makaroff, C. A. and Ma, H. (2003). The Arabidopsis MALE MEIOCYTE DEATH1 gene encodes a PHD-finger protein that is required for male meiosis. Plant Cell 15, 1281-1295. Yu, H. G., Muszynski, M. G. and Kelly Dawe, R. (1999). The maize homologue of the cell cycle checkpoint protein MAD2 reveals kinetochore substructure and contrasting mitotic and meiotic localization patterns. J. Cell Biol. 145, 425-435. Zeng, X. and Saunders, W. S. (2000). The Saccharomyces cerevisiae centromere protein Slk19p is required for two successive divisions during meiosis. Genetics 155, 577-587. Zhao, D. Z., Wang, G. F., Speal, B. and Ma, H. (2002). The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev. 16, 2021-2031.