* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Morphological Basis for the Cytolytic Effect of
Survey
Document related concepts
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Tissue engineering wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell culture wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
List of types of proteins wikipedia , lookup
Transcript
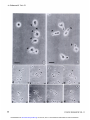
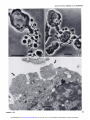
[CANCER RESEARCH 35, 497-501, March 1975] Morphological Basis for the Cytolytic Effect of Vinbiastine and Vincristine on Cultured Human Leukemic Lymphoblasts' Awtar Sidney Krishan and Emil Frei, Ill Farber Cancer Center and Harvard Medical School, Boston, Massachusetts 021/5 cultured human leukemic lymphoblasts cell line. SUMMARY Exposure of human leukemic lymphoblasts in suspension cultures to low concentrations of vinblastine and vincristine results in alterations in cell shape and leads to the formation and release of a large number of membrane-lined vesicles from the cytoplasm. Separation of these vesicles from peripheral cytoplasm is effected through alignment and fusion of small vacuoles. Similar vesicle formation is seen neither in fibroblasts exposed to vinblastine nor in lympho blasts exposed to bleomycin or adriamycin. Possible rela tion of this phenomenon to vinblastine- and vincristine induced cytotoxicity, spherocytosis, and thrombocytosis is discussed. INTRODUCTION Morphological alterations induced in mammalian cells by the ymca alkaloids, vinblastine and vincristine sulfate, have been well documented (5, 8-12). In low concentrations, these alkaloids bind to microtubular proteins causing depo lymerization of spindle microtubules and the resulting dissolution of the mitotic spindle (5, 8, 12). In cells exposed to high concentrations, large eosinophilic crystals (I, 10) and ribosomal complexes (aggregates of ribosomes and tubulin-like material) are seen in the cytoplasm ( I I). In monolayer cultures of Earle's L-929 mouse fibroblasts exposed to low vinblastine concentrations, initial arrest of MATERIALS of the CCRF-CEM AND METHODS Log-phase stock cultures of human leukemic lympho blasts, CCRF-CEM, which were initially isolated from the peripheral blood of a pediatric leukemic patient (4), were grown in roller bottles and nourished with Eagle's minimal essential medium (for spinner cultures) supplemented with 10% fetal calf serum, penicillin, and streptomycin. Earle's L-929 mouse fibroblasts and HeLa cells were grown in monolayer cultures and fed Eagle's minimal essential medium supplemented with fetal calf serum and antibiotics as above. Vinblastine sulfate (Velban) and vincristine sulfate (Oncovin) were obtained from Eli Lilly & Co., Indianapolis, Ind. For light-microscopic examination, cells were incubated in Sykes-Moore chambers and photographed under a Nikon inverted microscope fitted with time-lapse cinematographic equipment. For electron-microscopic examination, cell buttons re trieved after light centrifugation were fixed in 2% 0.1 M phosphate-buffered glutaraldehyde (pH 7.2), postfixed in 2% buffered osmium tetraoxide, dehydrated in a graded alcohol series, and embedded in an Epon-Araldite mixture. Ultrathin sectionswere stained with lead citrate and uranyl acetate and examined in a Philips 300 electron microscope. cells in mitosis is followed by a reorganization of chromo somes into micronuclei (8) and the formation of large, multimicronucleated cells. In contrast to this, exposure of cultured human lymphoblasts of the CCRF-CEM cell line RESULTS Morphological alterations reported in the present study were seen in both vinblastine- and vincristine sulfate-treated pronounced cytolysis of both the mitotic-arrested and CCRF-CEM cells. However, for the sake of brevity, we interphase cells. In the course of these observations, we have included observations only from the vinblastine noted pronounced alteration in the cell surface activity of treated cells. the drug-treated lymphoblasts and the release of numerous In CCRF-CEM cultures exposed to vinblastine (0.1 spherical vesicles from the peripheral cytoplasm of these @zg/ml)for 24 hr, approximately 50% of the cells were dead cells. In view of the possible connection between these or pyknotic. In contrast to this, Earle's L-929 and HeLa observations and earlier reported vinblastine-induced sphe cells similarly treated had less than 10% dead or pyknotic rocytosis (7) and thrombocytosis (3, 6, 14), we have cells. The pronounced cytolytic effect of these low drug examined in the present report the structural basis for the concentrations was seen in both interphase and mitotic pronounced cytolytic effect of vinblastine and vincristine on arrested CCRF-CEM lymphoblasts. Although the percent age of cells arrested in mitosis by vinblastine or vincristine was approximately similar in L-929 and CCRF-CEM cells, C-6516. Received September 20. 1974: accepted November 14, 1974. multimicronucleated cells (formed after reconstruction of (4) to similar low concentrations of vinblastine results in . a These MARCH studies were supported by National Cancer Institute Grant 1975 Downloaded from cancerres.aacrjournals.org on June 18, 2017. © 1975 American Association for Cancer Research. 497 A . Krishan and E. Frei, III nuclei in mitotic-arrested CCRF-CEM cultures. cells) were rarely seen in the Effect on Cell Shape and Pseudopods. Exposure of CCRF-CEM lymphoblasts to low concentrations of yin blastine (0.01 to 0.1 .ig/ml) for 30 mm led to alterations in both their external morphology and pseudopodal activity. In Fig. 1, lymphoblasts from a log-phase suspension culture are seen as more or less spherical cells with small pointed pseudopods and occasional ruffle membranes projecting from their leading edges (arrows). In contrast to this, lymphoblasts exposed to vinblastine (0.01 @g/ml)for 30 mm and shown in Fig. 2 have lost their spherical cell shape and, instead of the numerous small pointed pseudopods, have a single large clubfoot-like pseudopod projecting from one end ofthe cell (arrows). Fig. 3 shows a time-lapse sequence on the formation and behavior of these large pseudopods in a lymphoblast population exposed to vinblastine (0.05 sg/ml) for approxi mately 1 hr. The numbers on the right side of the frames indicate mm after the addition of vinblastine to the medium. The first (A) and the last (H) frames were photographed after 5 1 and 70 mm of exposure to the drug. Solid arrows in Frames A and D point to the formation and elongation of the large foot-like cytoplasmic projection from a lympho blast. In time-lapse movies, these foot-like cytoplasmic projections show an unusual tendency of sticking either to the substrate, in which case the rest of the cell rotates around a central fixed anchor-like attachment, or to similar processes from other cells. The latter process results in the formation of large cell clumps normally not seen in CCRF-CEM lymphoblasts growing in suspension cultures. In time-lapse Frames B to G of Fig. 3, formation of such a large cell clump in responseto drug exposureand the final lysis of the drug-treated cells is shown (Frames G and H). Formation and Release of Cytoplasmic Vesicles. Besides the above-mentioned morphological changes, CCRF-CEM lymphoblasts exposed to vinblastine or vincristine concen trations of 0.01 sg/ml show numerous cytoplasmic vesicles either in association with cells or floating freely in the medium. A wide variation in the size range of the observed cytoplasmic vesicles was evident; while some were as large as 5 @imacross, many others were at the limit of microscopic resolution. In cell cultures mixed with trypan blue or nigrosin black for cell viability determination, no dye uptake was seen in the vesicles, thus suggesting the presence of a dye-excluding cytoplasmic membrane. Free-floating cytoplasmic vesicles were seen in culture after as short a time as 2 hr of incubation with drug concentrations of 0.01 to 10 zg/ml. Similar cytoplasmic vesicleswere not seenin monolayer cultures of L-929 mouse fibroblasts or HeLa cells exposed to vinblastine or vincristine or in cultures of CCRF-CEM lymphoblasts exposed to bleomycin (1 to 100 @ig/ml) or adriamycin (1 to 10 zg/ml). Fig. 4 shows a lymphoblast from a culture exposed to vinblastine (0. 1 @ig/ml)for 6 hr. This photomicrograph was taken from a Sykes-Moore chamber preparation and shows numerous cytoplasmic vesicles being released from the peripheral cytoplasm of the cell. Some of the larger cytoplasmic vesicles contain granules that may be mito 498 chondria or lipid granules. This drug-induced cytoplasmic vesicle formation was seen in both nonmitotic interphase cells and in cells arrested in mitosis through the stathmoki netic action of the drug. In Fig. 5, a number of cytoplasmic vesicles released from a mitotic-arrested cell is seen. In electron micrographs these drug-induced cytoplasmic vesicles are seen as cell membrane-lined, free-floating portions of the ectoplasm, containing no major cellular organelles other than ribosomes and some small vacuoles. Fig. 6 shows a lymphoblast from a culture exposed to vinblastine (0.1 @tg/ml)for 24 hr. Arrows point to cytoplas mic vesicles at different stages of their separation and release from the peripheral cytoplasm of the lymphoblast. One of the cytoplasmic vesicles is nearly separated from the rest of the cell by a row of empty-looking vacuoles, whereas a 2nd vesicle is partially separated from the rest of the nucleus-containing cytoplasm by a row of vacuoles on either end. In time-lapse movies, as seenin Fig. 3 (Frames G and H), release of these vesicles from peripheral cytoplasm is followed ultimately by the bursting ofthe remaining nucleus or chromosome-containing cytoplasm, while the cytoplas mic vesicles float free in the medium and retain their identity for some time. DISCUSSION At the outset we must distinguish between the process of cytoplasmic vesicle formation as described in the present study and that of cytoplasmic blebbing or zeiosis seen occasionally in tissue culture cells (I 5). The cytoplasmic vesicle formation, as seen in lymphoblasts exposed to vinblastine, is similar to the process of platelet formation and releasein megakaryocytes(2), whereassurfaceblebbing of cultured cells, especially seen at anaphase, does not involve formation and alignment of small vacuoles or result in generation and release of small cytoplasmic vesicles. Surface blebbing induced in interphase cells (but not in@ mitotic cells) by exposure to hypotonic solutions has been shown to be dependent on the presence of Ca@ on the cell surface ( I 3). A calcium-mediated gelation differential is maintained in the cytoplasm of interphase cells, and an inward shift of Ca@ during mitosis is responsible not only for the breakdown of this differential but also possibly leads to chromosomal condensation ( I 3). In view of observations showing that vinblastine in higher concentrations acts like Ca@@and precipitates a number of cellular proteins as well as calf thymus DNA (16), the possible effect of vinblastine and vincristine on ectoplasmic gelation and cell membranes is not unexpected. In fact, such an effect has been demon strated in vinblastine-induced spherocytes, which resemble those of hereditary spherocytosis in their structure, osmotic fragility, and enhanced sodium flux rates (7). In morpholog ical studies, Jacob et al. (7) have shown that short-term exposure of erythrocytes from normal individuals to yin blastine leads to the formation of numerous cup-and-bowl shaped cells that, by fusion of their uninvaginated mem branes, form microspherocytes. Although this process of CANCER RESEARCH Downloaded from cancerres.aacrjournals.org on June 18, 2017. © 1975 American Association for Cancer Research. VOL. 35 Action of Vinca Alkaloids “endocytosis―differs morphologically from that of “ex ocytosis― seen in the present study, the basic mechanism responsible for the 2 processes may be similar and may involve either 1 or both of the following effects of vinblas tine on cells. (a) Vinblastine reduces the structural integrity of cyto plasm and this is reflected in drug-induced spherocytosis and formation and release of cytoplasmic vesicles from lymphocytes. Observations showing that higher concentra tions of colchicine (7) will causesimilar effectsand that cells growing attached to a substrate, as in monolayer cultures, do not show vesicle formation support this view. (b) The other profound effect of vinblastine may be exercised through its action on cell membranes. The in creased influx and efflux of sodium in erythrocytes (7) and enhanced transport of methotrexate in cells exposed to vinblastine have already been reported (18). Similar mem brane alterations are suggested by the effect of vincristine on calcium transport and phospholipid composition of skeletal muscle microsomes ( I7). Present observations may also be related to vinblastine and vincristine-induced thrombocytosis reported in both human clinical cases(3, 6) and in experimental animals (14). In rats given injections of a single dose of vincristine, pronounced thrombocytosis is seen on the 5th day and this effect is not abolished in either hypersplenic or splenecto mized rats, thereby ruling out the possibility of drug induced platelet release from the spleen (14). In view of the present observations, it is possible that thrombocytosis induced by vinblastine and vincristine may be caused by premature release of platelets from megakaryocytes through a mechanism similar to that described in the present report. On the basis of previous studies showing that the stathmokinetic effect of vinblastine and vincristine does not account for the selective cytocidal action of these com pounds and that fibroblasts exposed to vinblastine and vincristine do not show similar vesicle formation, we suggest that this phenomenonis drug specific and may be related to the pronounced lymphoblasts. cytocidal action of these alkaloids on REFERENCES 1. Bensch, K. G., and Malawista, S. E. Microtubular Mammalian Cells. J. Cell Biol., 40: 95-106, 1969. Crystals in on Lymphoblasts 2. Bloom, W., and Fawcett, D. W. A Textbook of Histology, Ed. 9, pp. 192- 194, Philadelphia: W. B. Saunders Co., 1970. 3. Carbone, P. P., Bono, V., Frei, E., III, and Brindley, C. 0. Clinical Studies with Vincristine. Blood, 2/: 640-647, 1963. 4. Foley, G. E., Lazarus, H., Farber, S., Uzman, B. G., Boone, B. A., and McCarthy, R. E. Continuous Culture of Human Lymphoblasts from Peripheral Blood of a Child with Acute Leukemia. Cancer, /8: 522-529,1965. 5. George, P.. Journey, L. J., and Goldstein, M. N. Effect of Vincristine on the Fine Structure of UcLa Cells during Mitosis. J. NatI. Cancer Inst., 35: 355—375, 1965. 6. Hwang, Y. F., Hamilton, H. E., and Sheets, R. F. Vinblastine-induced Thrombocytosis. Lancet, 2: 1075- 1076, 1969. 7. Jacob, H., Amsden, T., and White, J. Membrane Microlilaments of Erythrocytes: Alteration of Intact Cells Reproduces the Hereditary Spherocytosis Syndrome. Proc. Natl. Acad. Sci. U. S., 69: 47 1-474, 1972. 8. Krishan, A. Time-lapse and Ultrastructure Studies on the Reversal of Mitotic Arrest Induced by Vinblastine Sulfate in Earle's L-cells. J. Natl. Cancer Inst., 41: 581-595, 1968. 9. Krishan, A. Ribosome-Granular Material Complexes in Human Leukemic Lymphoblasts Exposed to Vinblastine Sulfate, J. Ultra structure Res., 31: 272-281, 1970. 10. Krishan, A., and Hsu, D. Observations on the Association of Helical Polyribosomes and Filaments with Vincristine-induced Crystals in Earle's L-cell Fibroblasts. J. Cell Biol., 43: 553-563, 1969. II. Krishan, A., Hsu, D., and Hutchins, P. Hypertrophy of Granular Endoplasmic Reticulum and Annulate Lamellae in Earle's L-cells Exposed to Vinblastine Sulfate. J. Cell Biol., 39: 21 1-216, 1968. 12. Malawista, S. E., Bensch, K. G.. and Sato, H. Vinblastine and Griseofulvin Reversibly Disrupt the Living Mitotic Spindle. Science, 160:770-772,1968. 13. Robbins, E., and Micali, A. The Use ofOsmotic Shock in the Study of the Mammalian HeLa Cell Surface Changes during Mitosis with Special Reference to Calcium-Containing Solutions. Exptl. Cell Res., 39:81-96, 1965. 14. Robertson, J. H., Crozier, E. H., and Woodend, B. E. The Effect of Vincristine on the Platelet Count in Rats. Brit. J. Haematol., 19: 331-337, 1970. 15. Rose, G. G. Atlas of Vertebrate Cells in Tissue Culture, pp. 36-40 New York: Academic Press, Inc., 1970. 16. Wilson, L., Bryan, J., Ruby, A., and Mazia, D. Precipitation of Proteins by Vinblastine and Calcium Ions. Proc. Natl. Acad. Sci. U. S., 66: 807-814, 1970. 17. Yasin, R., Hughes, B. P., and Parker, J. A. The Effect of Vincristine on the Calcium Transport and Phospholipid Composition of Rat Skeletal Muscle Microsomes. Lab. Invest., 29: 207-215, 1973. 18. Zager, R. F., Frisby, S. A., and Oliverio, V. T. The Effects of Antibiotics and Cancer Chemotherapeutic Agents on the Cellular Transport and Antitumor Activity of Methotrexate in Ll210 Murine Leukemia. Cancer Res., 33: 1670-1676, 1973. Figs. I and 2. Normal and vinblastine-treated lymphoblasts of CCRF-CEM cell line. Arrows in Fig. I show the pointed pseudopods and ruffle membranes of normal cells that are replaced in drug-treated cells by large foot-like projections (Fig. 2, arrows). Marker, 20 .im. Fig. 3 shows a time-lapse sequence of lymphoblasts exposed to vinblastine (0.05 @g/ml). Numbers in the right corner of each frame indicate mm of exposure to the drug. Arrows in Frames A to D show the formation ofa large cytoplasmic foot-like projection. Aggregation ofcells into clumps and the subsequent release of cytoplasmic vesicles is seen in Frames D to H. Figs. 4 and 5 show the formation and release of numerous cytoplasmic vesicles from the peripheral cytoplasm of an interphase cell (Fig. 4) and a mitotic-arrested cell (Fig. 5) exposed to vinblastine. Marker, 20 gzm. Fig. 6. Arrows in this electron micrograph point to the formation of cytoplasmic vesicles from the peripheral cytoplasm of a lymphoblast exposed to vinblastine. Rows of small vacuoles probably cause the release of the cytoplasmic vesicles by their alignment and fusion. Marker, I @.tm. MARCH 1975 499 Downloaded from cancerres.aacrjournals.org on June 18, 2017. © 1975 American Association for Cancer Research. @ .@ A . Krishan and E. Frei, III @ . Ii @ ..-( 1@ ( 0 ‘0 @ ‘ ‘ : - p. 1@ . @ @ ,-.“ ‘V . a F— . : I @*,.@- .1 1% it ..‘ ‘p •@ , , . A ...‘ . .T . I .@ 52C@ 51 454D 5t -.@ @. E,.-@ • . .‘9@ @S,! a @4, Es 500 ‘..-#@-- - :.@ - - •: , @0—@ .-@. I I -F .@- % 62 @. ; @-i S 66e.'-:@“@•‘:: “6SF! --II. CANCER RESEARCH 70 VOL. 35 Downloaded from cancerres.aacrjournals.org on June 18, 2017. © 1975 American Association for Cancer Research. Action of y inca Alkaloids on Lymphoblasts ' ', MARCH - 1975 Downloaded from cancerres.aacrjournals.org on June 18, 2017. © 1975 American Association for Cancer Research. 501 Morphological Basis for the Cytolytic Effect of Vinblastine and Vincristine on Cultured Human Leukemic Lymphoblasts Awtar Krishan and Emil Frei III Cancer Res 1975;35:497-501. Updated version E-mail alerts Reprints and Subscriptions Permissions Access the most recent version of this article at: http://cancerres.aacrjournals.org/content/35/3/497 Sign up to receive free email-alerts related to this article or journal. To order reprints of this article or to subscribe to the journal, contact the AACR Publications Department at [email protected]. To request permission to re-use all or part of this article, contact the AACR Publications Department at [email protected]. Downloaded from cancerres.aacrjournals.org on June 18, 2017. © 1975 American Association for Cancer Research.