* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Some effects of environment and hormone treatment on
Photosynthesis wikipedia , lookup
Gartons Agricultural Plant Breeders wikipedia , lookup
History of herbalism wikipedia , lookup
Plant tolerance to herbivory wikipedia , lookup
History of botany wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Plant nutrition wikipedia , lookup
Plant breeding wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Flowering plant wikipedia , lookup
Plant stress measurement wikipedia , lookup
Venus flytrap wikipedia , lookup
Plant physiology wikipedia , lookup
Plant ecology wikipedia , lookup
Plant reproduction wikipedia , lookup
Sustainable landscaping wikipedia , lookup
Plant morphology wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
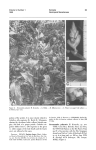
254 W. W. SUHWABE: [J.L.s.B. LVI Some effects of environment and hormone treatment on reproductive morphogenesis in the Chrysanthemum. By W. W, SOHWABE.Research Institute of Plant Physiology, Imperial College, London (With Plate 10 and 2 Text-figures) INTRODUUTION The study of morphogenesis in the plant is mainly concerned with the events at the growing points. I n the higher plants, in the vegetative condition, the active stem apices give rise to the leaves and their associated lateral buds, and their initiation and development follow a regular sequence which, under normal circumstances, is not easily disruptedexcept by surgical and other drastic techniques. The positioning of the young primordia, their size at initiation relative to the main growing point and their rate of growth, determine the phyllotactic pattern and also to some extent the general appearance of the plant. When the plant becomes reproductive there is a change to an entirely different sequence which is equally regular and fixed. I n contrast to the leaf, the flower primordium represents an axial structure and in many instances it may be regarded as a precocious lateral bud. While the onset of reproduction frequently leads to the formation of modified leaves or bracts before the actual flower primordia arise, this is only a very transitory phase and each growing point must follow either one or other main sequence, and it is not possible for it to persist in producing intermediate structures. This in itself indicates that when reproduction starts, there is a complete switch-over at the apex from one morphogenetic pathway to another. It is this fact which has also served to justify the concentration of research on the actual ‘initiation’ of flowering; clearly it is ‘le premier pas qui coGte’. Once reproductive development has begun, the growing point affected must either continue this new line of development or f i s h ; only in relatively rare teratological instances is a return to the earlier sequence possible. Experimentally, it is, of course, possible to modify the various stages of floral or reproductivedevelopment also, but under normal conditions this does not usually take place. Confining attention to the Chrysanthemum, firat consideration is to be given to what is known about the behaviour of the vegetative apex, which factors of the environment, etc., are known to affect the transition from the vegetative line of development to the reproductive, and what changes have been observed to occur at the apex during this stage. Finally, the factors influencing the later stages of reproductive development will be discussed. It must be stressed, however, that in a plant of such horticultural interest as the Chrysanthemum large numbers of different varieties have been selected-often specifically for differences in their physiological behaviour. Hence, varietal differences in response must be pronounced and not all the factors to be discussed will be of equal importance in all strains. I n most of the experimental work to be described, the variety ‘Sunbeam’ was used, and it is believed that this variety is relatively unselected and perhaps nearer to the ‘wild type’ of Chrysanthemum than many other varieties. A. THE VEGETATIVE GROWING POINT The vegetative growing point of the Chrysanthemum is seen in PI. 10, after all young leaf primordia have been cut away. The bare apex, unoccupied by such primordia, is approximately 1 6 0 , in ~ diameter, and its mean area remains relatively constant throughout the period of vegetative growth. During this period it cuts off leaf initials a t a regular rate. At initiation the area of each primordium is approximately equal to the transverse area of the bare apex itself as determined by Evington (1964),using the methods evolved by Riahards (1948, 1981). J.L.S.B. LVI] REPRODUCTIVE MORPHOGENESIS I N THE CHRYSANTHEMUM 255 The rate of primordium formation during vegetative growth may vary from 2.2 to 3.2 per week, i.e. defining the rate of initiation at the apex as the 'true plastochron ' this varies from 3.2 to 2.2 days. The variation is due to the fact that the previous history of the plant and also the prevailing environment can affect this rate. The effects of a variety of factors and their combinations have been investigated; one of the most important being the temperature history of the plant, i.e. whether it has been vernalized or not. Determinations of the 'true plastochron' for plants grown in identical conditions, but of different temperature history yielded the following values : Unchilled control 2 weeks chilling 4 weeks chilling 3-18f 0.18 days 2.44 0.13 days 2.26 f0.18 days The actual growth temperature does not appear to affect the 'true plastochron' very much, regardless of whether the temperature is held constant or whether different day and night temperatures are given, as will be seen below from the data of two experiments: Constant day and night temperature 17' C., 2-79 d ~ y s 22" C., 2.54 days 27" C., 2.69 days Day temperature 17" C., 2-25 days 22" C., 2.14 day8 27" C., 2-25 days Night temperature 17"C., 2-29 days 22" c., 2.25 days 27" C., 2.10 days This relative independence of temperature applies, however, only t o the 'true plestochron', i.e. the actual rate of leaf primordium initiation as determined by dissection of the buds. The 'apparent plastochron', i.e. the rate of unfolding of new leaves from the bud, as determined by leaf counts, is affected very much more by the actual growth temperature, and the following values were determined in one experiment : 18"C., 3.86 day6 22" C., 3.02 days k0-18 26" C., 2.61 days The marked difference between these two measures is due to the fact that at lower temperatures more leaves are accumulated in the terminal bud which then do not expand as rapidly as they are initiated. Internally there is, of course, some heat production in the bud, and the apex within it is also relatively well insulated by layers of air between the primordia. Therefore, a mechanism retarding expansion of the outer primordia might perhaps serve as a homoeostatic regulator allowing the growing point itself to be a t a somewhat different temperature from the outer environment. However, this suggestion is purely speculative, and in fact it seems doubtful whether it could quantitatively account for the observed effect. Before leaving the discussion of the behaviour of the vegetative apex, brief reference may be made to the fact that the morphology of the young primordia themselves is also determined a t an early stage in the bud. The external environment is also capable of modifying their final shape while acting at an early stage in their ontogeny. A good example of this is seen in the temperature effect on leaf shape. I n the Chrysanthemum the degree of dissection varies very much with the temperature prevailing during initiation. Leaves initiated at relatively high temperature (27' C.) become very much less dissected than those formed at a lower temperature (17" C.). (Text-fig. 1.) I B. THE CHANGE-OVER TO THE REPRODUCTIVE CONDITION (i) Effects of the environment. In the Chrysanthemum, as in so many other plants, the onset of reproductive development is largely subject to control by the environment. The actual conditions required have been fairly closely studied and at least for the Text-fig. 1. Temperature effect on leaf shape. Leaves arranged in order of their ages. Left: from a plant grown at 27" C.; right: from a plant grown at 17' C. .. J.L.S.B. LVI] REPRODUCTIVE MORPEOQENESIS IN THE UHRYSANTHEMUM 257 variety ‘Sunbeam’ are now known in considerable detail. It must be stressed, however, that other Chrysanthemum varieties are likely to show at least quantitative differences from the optimum valves established. For brevity’s sake, the various factors controlling the switch-over to the reproduction condition and its continuation have been summarized in tabular form (Table l), which also shows the effective ranges of duration and level. Table 1. Sequence of factors controlling the initiation of reproductive development in the Chrysanthemum,and their optimum levels and duration Factor Low temperature (vernalization) Growth temperature Day Night Long-day treatment Short-day treatment I Light intensity during growth period (Variety ‘Sunbeam’) Level Duration 1°-120 c. 3-4 weeks 22”-23” C. Until flowering Schwabe, Unpubl. 3-4 weeks From then on till flowering > Approx. 1600-2000 f.c. Throughout 16 hr. day 8-10 hr. day Reference Schwabe, 1960 I Schwabe, 1952a Schwabe, 1962b This table represents, of course, an oversimplification,since there are numerous interactions between the various factors. As regards their seat of action, it is also known that those conditions involving the light factor, i.e. light intensity and duration, etc., are perceived by the leaves. On the other hand, grafting experiments and experiments in which different parts of the plants were subjected to different temperature conditionshave shownthat the vernalizationeffectis perceived in thegrowingpoint itself.The physiological changes wrought there persist only in the meristem and are not translocated about the plant through mature tisaue, but they may increase in parallel with apical growth and in this way be passed on to newly arising axillary meristems (Schwabe, 1954). (ii) Internal and nutritional factors. Not much is known as yet about the internal factors involved in the onset of flowering. Nitrogen content, as controlled by the level of this nutrient in the culture medium, has only a slight effect by accelerating or, when deficient, retarding growth generally. In one experimentnitrogen-deficientplants budded a mere 4 days earlier than the controls with full nutrient supply, and the number of leaves produced prior to bud formation was actually lower than in the controls. Also chromatographic analysis of the growing points of vegetative and budded plants did not reveal any marked differences in the free amino acids present. Carbohydrate level also seems to be of little importance for the vernalization processes accelerating flowering in this plant. This was revealed in some experiments on the devernalization of the Chrysanthemum. Such de-vernalization can be achieved by prolonged exposure to low light intensity treatment (Schwabe, 1957b), a treatment which serves, of course, to deplete the carbohydrate reserves of the plant very severely indeed. In plants so treated stem growth and leaf expansion are brought almost to a complete halt. Nevertheless, such starved plants can be completely re-vernalized a t once by a second chilling period while still in the starved condition. Also, while application of soluble carbohydrate to such plants will permit stem growth and leaf expansion to continue, it will not avert the de-vernalizing effect. Hence the role of carbohydrate in the vernalization process is at best subordinate. (iii) Hormones. As regards hormones there is now some evidence that their application may delay or hasten the transition to the flowering condition. The initiation of reproductive development may be delayed by indole-3-aceticacid in lanolin paste if this is applied repeatedly just below the terminal growing point, even when the plant ia fully vernalized. In one experiment six such applications of paste delayed bud formation by 258 W. W. SURWABE: [J.L.s.B. LVI three weeks and caused the auxin-treated plants to produce on the average an additional six leaves before budding. Gibberellic acid? on the other hand, applied in solution to the young leaves was shown t o induce a proportion of plants to flower even in the absenoe of any chilling treatment, as will be seen from Table 2, but the effect waa very slight, when leaf number figures are considered, compared with that of vernalization. Other synthetic growth substances auch as triiodobenzoic acid do not appear to affect the onset of the reproductive phase to any significant extent. (iv) Changes at the apex itself. One of the most striking changes at the apex during the transition to the flowering state is the enormoua increase in the size of the bare, uncommitted apex itself. From a diameter of approximately 160p, in the vegetative condition, the apex increases to approximately 3000p in diameter at the time just before floret initiation, a 399-foldincrease in area. Although the apex increasesgradually during growth most of this change in apical size occurs over the relatively short period of a few days. Table 2. Effect of gibberellic acid on jlowering of vernalized and unvemzalized Chy8anthmum plants (Variety ‘ Sunbeam’. Ten replicates.) Proportion Treatment budded Unvernctlized: 0 p.p.m. gibberellic mid (control) 10 p.p.m. gibberellic mid (2 c.c.) 100 p.p.m. gibberellic mid (2 c.c.) Vernalized : 0 p.p.m. gibberellio mid (control) 10 p.p.m. gibberellic mid (2 c.c.) 100 p.p.m. gibberellic mid (2 0.0.) 0/10 1/10 4/10 lop0 10/10 lop0 *Leaf number to budding - (37.0) 49 26.0 26.3 27.0 * Leaf number of vegetative plants at end of experiment in parenthewe. Text-fig. 2. Transition from foliage leaves to inflorescence brmta. Kindly donated by Dr P. W. Brian. (38.8) 40.8 (35.1) J.L.S.B. LVI] REPRODUCTIVE MORPROUENESIS IN THE CHRYSANTHEMUM 259 Simultaneously, the other well-known changes take place ; the type of primordium initiated changes and after a small number of rather reduced leaves have been formed the floral bracts areproduced (Text-fig.2). At this stage there is a large and in thechrysanthemum quite rapid rise in the order of phyllotaxis. Measured in Richards’s (1951) equivalent phyllotaxis index, the index is about 2.1 for the vegetative apex, and rises by 1.8 units in the reproductive. Also the time required to initiate these inflorescence bracts is reduced very considerably. Whereas, as was seen above, vegetative plants require 2.2 and 3.2 days in the vernalized and unvernalized state respectively for the initiation of each leaf, a rough average plastochron for inflorescencebracts is approximately 9 hr. To estimate such times is not very easy technically, but fortunately the terminal and upper lateral inflorescence buds of the Chrysanthemum form a more or less linear series of development, the uppermost being the most advanced. If the top and bottom members of such a set of three successive buds on a plant are dissected and the number of primordia in them determined, then a good estimate is obtained of the number of primordia present in the intermediate bud. Dissection of this bud after a further period of 1-2 days or even hours yields an estimate of the number of primordia initiated in the interval. In the variety ‘Sunbeam’ about thirty such bracts are formed. Their size, especially their thickness, becomes progressively reduced, and the ‘blades ’ of the innermost bracts are only two cells thick, any further reduction in the radial dimension would seem impossible and under ordinary circumstances bract production then ceases abruptly and floret production commences. It is an interesting fact, which may well be associated with the great reduction of the radial dimension of these primordia, that these bracts which arise round the extreme edge of the enlarging bare apex carry no lateral bud initials in their axils at all. C. THEREPRODUCTIVE UROWINU POINT The actual moment when the apex is to be regarded as definitely reproductive might be defined differently according to the problem under consideration. However, in general it would seem to be most satisfactory to define it as the point when any further development of it must be reproductive, i.e. when the change has become irreversible. In the Chrysanthemum this is the moment when the apex has enlarged and the receptacle is formed. The normal sequence after this concerns only the formation and development of the florets and their ultimate functioning. As the initiation of the florets themselves begins (i.e. primordia representing axial structures) the large bare apical area is invaded and very soon used up altogether, thus terminating the growth of the original growing point. The rate of such primordial initiation has also been estimated for the outer florets in the same manner as described above for the bracts, though, of course, with a correspondingly greater margin of error. The time for the initiation of each of these florets amounts to about 24 min. under approximatelyoptimum greenhouse conditions. At this rate about 6Ofloretsare formed in one day, and since the inner florets are probably formed even faster, about 3-4 days will suffice to initiate the usual 200-300 florets in the bud and conclude this phase. The equivalent phyllotaxis index at this stage is approximately 7. An apex in the process of initiating floret primordia is seen in PI. 10. However, the behaviour of the growing point, even after receptacle formation, is still subject to environmental control. Of the various factors investigated it appears that the light factor is the most important-acting via the leaves and leafy bracts. Two aspects of the light factor are of importance in this respect-the duration of photoperiod and also the intensity, and in unfavourable light conditions the whole sequence of development may be completely arrested. It seems most likely that these effects are indirect, acting through modification of the auxin metabolism of the plant. There are two lines of evidence for this: (a) any treatment causing an increase in auxin level appears to inhibit 260 W. W. SCHWABE: [J.L.s.B. LVI floral development; while (a) treatments causing a reduction of auxin level appear to overcome the inhibition. Very briefly the experimental evidence for this is as follows. Apices of plants forming a receptacle in long-day conditions cease to develop any further after this point has been reached, and no florets are formed ;the bare receptacle ends the existence of the growing point concerned (Pl. 10). Below it the next 3-4 lateral buds then grow out vegetatively, giving rise to new shoots which may again form abortive inflorescence buds. A further set of vegetative laterals will grow out below these buds and the cycle may be repeated again and again. Although the plant, as a whole, is thus prevented from becoming truly reproductive, the terminal growing point itself cannot revert to the vegetative condition. It appears that a t the moment the receptacle is formed the main apex loses its apical dominance and Unless this can be re-established after floret initiation dominance is assumed by the laterals below, which then stop further growth of the terminal apex. Similarly plants which have reached the stage of receptacle formation in short-day conditions may be inhibited from flowering by transfer to long day, but here the stage of development reached at the time of transfer is important. The latest stage at which long-day conditions can arrest the further development is that of gynoecium formation in the marginal florets. Although there is little positive evidence as yet which is really convincing, it does seem that in long days higher auxin levels are attained. In view ofthis, another treatment claimed t o lead to enhanced auxin effectswas applied to plants which had budded in the favourable short-day conditions. This treatment which consisted in a severe reduction of light intensity (to about 25 f.c.)while the plants remained in short days, also caused the suppression of further growth of the developing inflorescence in the same way as long days, and, moreover, the same limitation of effectiveness as regards the stage of development reached a t the beginning of treatment was observed. Buds which had passed this stage were not arrested. Finally, actual application of hormones (indole-3-acetic acid in lanolin paste) just below the inflorescence bud proved to be quite as effective as long days or reduced light in stopping development, although here the effect was of course limited to the bud so treated. Once again the stage of development reached was of importance and older buds continued their development even in the presence of auxin. Reduction of auxin level was achieved by transfer of plants from long days to short days, which allowed the arrested inflorescencebuds to recommencetheir development and to produce flowers, if they were still alive at this time. Also, removal of the main sources of the excess auxin produced in long days, i.e. the young vegetative laterals growing out below the bud, permitted further development of the terminal bud. This could be done either by complete disbudding of all laterals while the plant was maintained in long days, or by severing the arrested inflorescence bud itself, leaving on only a few bracts, and re-rooting it in long days. In all these instances inflorescenceswere produced. There was some evidence that the leaves or bracts still exerted in long days some slight inhibition, since floral development was even more rapid if the plants were transferred to short days in addition to the disbudding. However, with these treatments some abnormalities and teratological effects became apparent. Although the development of the terminal growing point as a whole remained entirely reproductive, small parts of it produced a little vegetative growth before again ending in an inflorescence. For instance, in such inflorescences the initiation of bracts was not suddenly halted a t the periphery of the receptacle, but continued until the whole of the original apex had become covered with florets. Most of such florets arose in the axils of bracts, much a~ in other members of the Compositae not included in the subfamily Chrysantheminae (in some Classifications) of which the Chrysanthemum is the type genus, and which is in fact characterized by the absence of such bracts. Another such observation was the appearance of secondary inflorescences from the W. W. SCHWABE Journ. Linn. SOC. Bot. Voi. LVI, Pi. 10 (Fucimg p . 261) J.L.S.B. LVI] REPRODUUTIVE MORPHOOENESIS IN TIIE CHRYSANTHEMUM 261 gynoecium of the marginal ligulate florets-like the hen-and-chicken daisy. A very similar effect in Helenium, which was recently brought to notice, was almost certainly due t o accidental spraying with a hormone weed killer. Even the individual florets may be modified in their morphology. Thus hormone treatment of the main bud frequently leads t o the production of tubular instead of ligulate florets in adjacent inflorescences (Pl. 10). Some varieties of the Chrysanthemum produce such ‘quill’ flowers quite normally, and it seems possible that these may differ from other varieties in their hormone levels at the susceptible stage. I n concluding this very brief survey of the morphogenetic activity of the Chrysanthemum apex, it may be said that there are two sequences of organ production: the vegetative, which is indeterminate and concerned with essentially similar structures throughout, and the reproductive, which so far as the individual apex is concerned, is determinate and is concerned with a sequence of structures showing a range of form. Both sequences in themselves are relatively stable and can be upset by drastic treatments only, such as application of hormones or surgical techniques. The change-over from one to the other condition is itself controlled by environmental factors and in the Chrysanthemum, a t least, the change-over appears possible only in one direction. Once the growing point has become reproductive it must complete this sequence or die prematurely. The actual change itself also follows a regular sequence. The great increase in apical size is associated with a related change in primordial size and possibly as a consequence their h a 1 shapes. When the radial diameter of the primordia is so small that no more bracts are produced, itself possibly a consequence of spatial factors, the bare apex is rapidly used up in the formation of the florets. It seems likely that in the Chrysanthemum, as in other plants, the change is controlled by hormones and probably a balanced system of promotion and inhibition is concerned. REFERENCES EVINOTON, D. G., 1954. A n investigation of Phyllotaxis end Apical Growth in Flax and Chrysanthemum. Ph.D. Thesis, London. RICHARDS, F. J., 1948. The geometry of phyllotaxis and its origin. Symp. SOC. Exp. Biol. 2, 217-45. RICHARDS, F. J., 1951. Phyllotaxis: its quantitative expression and relation to growth in the apex. Ph4. Trans. B, 235, 509-64. SCHWABE, W. W., 1950. Factors controlling flowering in the Chrysanthemum. I. The effects of photoperiod and temporary chilling. J. Exp. Bot. 1, 329-43. SCHWABE, W. W., 1952a. Effects of temperature, day length and light intensity in the control of flowering in the Chrysanthemum. Rep. X I I I t h Int. Hort. Congr. pp. 1-9. SCHWABE, W. W., 1952b. Factors controlling flowering in the Chrysanthemum. 111. Favourable effects of limited periods of long-day on inflorescence initiation. J. Ezp. Bot. 3, 430-6. SCHWABE, W. W., 1954. Factors controlling flowering in the Chrysanthemum. IV. The site of vernalization and translocation of the stimulus. J. Exp. Bot. 5 , 389-400. SCHWABE, W. W., 19570. The study of plant development in controlled environments. I n Control of tke Plant Environment (5. P. Hudson, ed.), pp. 16-35. London. SCHWABE, W. W., 1957b. Factors controlling flowering in the Chrysanthemum. VI. De-vernalization by low-light intensity in relation to temperature and carbohydrate supply. J . Exp. Bot. 8, 220-34. EXPLANATION O F PLATE PLATE10 (a) Bare apex (A) of vegetative Chrysanthemum plant after all leaf primordia have been cut away, except the youngest ( P ) . (b) Receptacle, formed in short day, in process of floret initiation. (c) Receptacle, formed in long day, and prested without floret formation. (d) Abnormal production of tubular florets instead of ligulate florets, caused by application of indole-3-acetic acid to the plant. Left : normal inflorescences; right : inflorescences with tubular florets.