* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 5 - Fermentation and CHO feeder
Metabolic network modelling wikipedia , lookup
Butyric acid wikipedia , lookup
Biochemical cascade wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Blood sugar level wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Microbial metabolism wikipedia , lookup
Phosphorylation wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
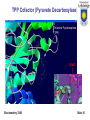
Department of Chemistry and Biochemistry University of Lethbridge Biochemistry 3300 III. Metabolism Glucose Catabolism – Part II Biochemistry 3300 Slide 1 Metabolic Fates of NADH and Pyruvate Cartoon: Fate of pyruvate, the product of glycolysis. +O2 -O2 Biochemistry 3300 TCA cycle Fermentation Slide 2 Metabolic Fates of NADH and Pyruvate Pyruvate is a central branch point in Metabolism. Aerobic pathway: Citric acid cycle and then respiration; - yields far more energy (discussed later) than glycolysis - relatively slow (limited by O2 transport) NADH + O2 → NAD+ + Energy Pyruvate + O2 → 3 CO2 + Energy Biochemistry 3300 Slide 3 Metabolic Fates of NADH and Pyruvate Pyruvate is a central branch point in Metabolism. Two anaerobic pathways: -Pyruvate is converted to lactate via lactate dehydrogenase (ie. muscle cells) -Pyruvate is converted to ethanol via ethanol dehydrogenase (ie. yeast) Anaerobic pyruvate utilization = Fermentation Both pathways use the NADH (produced in glycolysis): Overall: Glucose → 2 lactate + 2 ATP Biochemistry 3300 Slide 4 Lactate Fermentation Enzyme = Lactate Dehydrogenase Pyruvate + NADH + + H+ L-Lactate + NAD+ Regenerates NAD+ from NADH (reducing equivalents) produced in glycolysis. Essential as NAD+ is required for glycolysis (step 6 -GAPDH) Lactate fermentation is important in red blood cells, parts of the retina and in skeletal muscle cells during extreme high activity. Also important in plants and microbes growing in absence of O 2. ∆G’° = -25.1 kJ/mol Biochemistry 3300 Slide 5 Lactate Dehydrogenase (LDH) In mammals two different types of LDH subunits are found: the M type and the H type. Five forms of the tetrameric isozymes are possible: M4, M3H1, M2H2, M1H3, H4 H-type predominates aerobic tissues (ie. heart muscle) H4 LDH has a low KM for pyruvate and is allosterically inhibited by it. M-type predominates in tissue subject to anaerobic conditions (ie. liver and skeletal muscle) M4 LDH has a low KM for pyruvate and is NOT allosterically inhibited by it. Biochemistry 3300 Slide 6 Lactate Dehydrogenase (LDH) NADH LDH monomer NADH shown as sticks Catalytic site circled Redox reaction involving electron transfer from NADH to pyruvate. Biochemistry 3300 Slide 7 Reaction Mechanism of Lactate Dehydrogenase Biochemistry 3300 Slide 8 Pyruvate: Terminal Electron Acceptor of Lactic Acid Fermentation Fate of Lactate (from fermentation) Corey Cycle: Most lactate is exported from the muscle cell via the blood to the liver ↓ Liver converts lactate (back) to glucose ↓ Glucose is transported from liver cells via the blood to the muscle (stored as glycogen) The process of transporting lactate to the liver and its conversion to glucose takes from hours to days to complete. Biochemistry 3300 Slide 9 Alcoholic Fermentation Two enzymes involved: Pyruvate decarboxylase irreversible Alcohol dehydrogenase reversible Regenerates NAD+ from NADH (reducing equivalents) produced in glycolysis. Pathway is active in yeast Second step is reversible Ethanol can be further metabolised via oxidation that ultimately produces acetate and enters fat biosynthesis pathways Biochemistry 3300 Slide 10 Pyruvate Decarboxylase (Alcohol Fermentation) Yeast produces CO2 and ethanol in two consecutive reactions Decarboxylation of pyruvate to acetaldehyde is catalyzed by pyruvate decarboxylase (PDC) (not present in animals). PDC contains a tightly non covalently bound coenzyme: Thiamin pyrophosphate (TPP) Catalytically active Biochemistry 3300 Slide 11 TPP Cofactor (Pyruvate Decarboxylase) The dipolar carbanion (ylid) is the active form Decarboxylation of α-keto acids builds up negative charge on the carbonyl carbon. Transition state is stabilized by delocalization of the developing neg. charge into a “electron sink”. Biochemistry 3300 Slide 12 TPP Cofactor (Pyruvate Decarboxylase) Thiamine Pryophosphate (TPP) TPP of Pyruvate Decarboxylase: Two views related by 90º rotation about a vertical axis Biochemistry 3300 Slide 13 TPP Cofactor (Pyruvate Decarboxylase) How is TPP deprotonated to its the ylid form? 1) TTP’s aminopyradine ring (subunit 1) is deprotonated by Glu51 (subunit 2) of the PDC dimer. 2) amine of aminopyradine deprotonates thiazolium ring producing ylid form TPP Note: PDC is a dimer of dimers. Note: TPP ylid form circled in red Biochemistry 3300 Slide 14 TPP Cofactor (Pyruvate Decarboxylase) Thiamine Pyrophosphate (TPP) Glu51 Biochemistry 3300 Slide 15 Thiamine Deficiency TPP addition to carbonyl groups and its ability to act as an “electron sink”(electron withdrawl) makes it the coenzyme most utilized in α-keto acid decarboxylations. Thiamin (vitamin B1) is not synthesized or stored in significant amounts by vertebrates. Deficiency in humans results in an ultimately fatal condition known as beriberi. Biochemistry 3300 Slide 16 Alcoholic Fermentation (step II) Reduction of acetaldehyde to ethanol and regeneration of NAD + by alcohol dehydrogenase (ADH) Each subunit of the tetrameric yeast ADH binds one NADH and one Zn2+. Biochemistry 3300 Slide 17 Alcoholic Fermentation Part II Zn2+ polarises the carbonyl oxygen of acetaldehyde Hydride ion is transferred from NADH to the carbonyl carbon Reduced intermediate acquires a proton from the medium to form ethanol. Biochemistry 3300 Slide 18 Glycolysis: Substrates other than glucose Glycogen / Starch Dietary Polysaccharides Maltose (Glu-Glu) Lactose (Glu-Gal) Sucrose (Glu-Fru) Biochemistry 3300 Slide 19 Feeder Pathways for Glycolysis Glycogen metabolism Glycogen storage granules in liver Enzymes of 'feeder pathways' are underlined in red Biochemistry 3300 Slide 20 Phosphorolysis: glycogen / starch degradation Glycogen phosphorylase / Starch phosphorylase - attack of Pi on the (α1→4) glycosidic linkage of the last two glucose residues. Phosphorolysis generates G1P which must be converted to G6P (phosphoglucomutase) to enter glycolysis Biochemistry 3300 Slide 21 Phosphorolysis: glycogen / starch degradation Phosphorylase - repetitively breaks (α1→4) linkages until it reaches an (α1→6) - produces glucose-1phosphate Debranching enzyme - required to break (α1→6) linkages - produces glucose Biochemistry 3300 Slide 22 Phosphoglucomutase mechanism Glucose 1-phosphate has to be converted into glucose 6-phosphate to enter glcolysis Where have we previously seen this type of mechanism? Biochemistry 3300 Slide 23 Phosphoglycerate Mutase – Reaction 8 Similar mechanism to phosphoglycerate mutase (glycolysis) - different catalytic residue Biochemistry 3300 Slide 24 Complication! – The Liver Glycogen is primarily stored in the liver and is used to maintain blood glucose levels between meals But … neither G1P nor G6P can be transported out of liver cells Require separate pathway (below) to convert G6P to glucose for transport Biochemistry 3300 Slide 25 Dietary Polysaccharides Dextrin + n H20 → n D-glucose Dextrinase Maltose + H20 → 2 D-glucose Maltase Lactose + H20 → D-galactose + D-glucose Lactase Sucrose + H20 → D-fructose + D-glucose Sucrase Di- and polysaccharides are converted to monosaccharides, then funneled into the glycolytic sequence Biochemistry 3300 Slide 26 Fructose entry into Glycolysis Two routes for fructose entry into glycolysis - tissue specific Biochemistry 3300 Slide 27 Fructose entry into Glycolysis Non-Liver D-Fructose is phosphorylated by hexokinase and F6P enters glycolysis: Mg2+ Fructose + ATP → fructose 6-phosphate + ADP Liver D-Fructose phosphorylated by fructokinase (at C1): Mg2+ Fructose + ATP → fructose 1-phosphate + ADP Fructose 1-phosphate is then cleaved to glyceraldehyde and dihydroxyacetone phosphate (DHAP) by fructose 1-phosphate aldolase. DHAP and glyceraldehyde-3-phosphate Are both glycolytic intermediates Biochemistry 3300 Glyceraldehyde is phosphorylated by triose kinase and ATP to glyceraldehyde-3-phosphate. Slide 28 Galactose entry into the Glycolysis Galactose entry into glycolysis is more complex than for other dietary sugars Biochemistry 3300 Slide 29 Galactose conversion to Glucose-1-phosphate Metabolism of Galactose involves three enzymes and a sugar nucleotide. Glycolysis C1 carbon is activated as phosphate ester Biochemistry 3300 Textbook (3rd Edition) has typo that is corrected here Slide 30 Galactose conversion to glucose-1-phosphate UDP-glucose + Galactose-1-phosphate ↓ UDP-galactose + Glucose-1-phosphate Must regenerate UDP-glucose to continue cycle → glycolysis Activation of C1 phosphate via formation of phosphate ester with UDP Biochemistry 3300 Slide 31 Conversion of UDP-galactose to UDP-glucose Textbook (3rd Edition) has typo that is corrected here Biochemistry 3300 Slide 32