* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ultrastructural studies of t /t mouse embryos
Survey
Document related concepts
Cell nucleus wikipedia , lookup
Lipid bilayer wikipedia , lookup
Tissue engineering wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Endomembrane system wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cytokinesis wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Transcript
/. Embryo/, exp. Morph. Vol. 33, 3, pp. 685-695, 1975
Printed in Great Britain
685
Ultrastructural studies of tw32/tw32 mouse embryos
By NINA HILLMAN 1 AND RALPH HILLMAN 1
From the Department of Biology, Temple University, Philadelphia
SUMMARY
Homozygous t"' lt"' mouse embryos, obtained from both spontaneously ovulated and
superovulated T+lt"'32 females mated to T+jtu'32 males, have a lethal period which extends
from the 8-12 cell stage to the late morula stage. Most of the homozygous mutant embryos
die at the early morula stage and are characterized by excessive amounts of cytoplasmic lipid,
mitochondrial abnormalities, binucleated cells and nuclear lipid droplets. The excessive
cytoplasmic lipid and nuclear lipid droplets distinguish 35-50% of the embryos (presumably
f "32 homozygotes) from their litter-mates prior to the lethal period. The remainder of the
distinguishing characteristics appear in the later (8-cell to late morula) tw32jtw3i embryos in
frequencies high enough to be considered phenotypic expressions of the mutant genome. The
present study indicates that the non-complementary tu'32 and t12 alleles are in fact separate
T locus recessive alleles.
32
32
INTRODUCTION
The complex TJocus is located on linkage group IX (chromosome 17) of the
house mouse. The homozygous T\T genotype is lethal at gestation days 10-11
(Chesley, 1932) while the heterozygous genotype T+jT is viable but results in
offspring with a short-tail phenotype. The dominant allele, T, and a series of
recessive lethal alleles (tn) are maintained in balanced lethal lines (Tftn) which
are tailless. Two of these recessive alleles, tw32 and t12, have been assigned to the
same complementation group of the T locus (Bennett & Dunn, 1964). This
assignment is a result of studies which show that the heterozygous twZ2/t12
embryos die at the late morula stage, as do the tw32 and the t12 homozygotes.
Also, both tu'32 homozygotes and twS2/t12 heterozygotes show, at the light
microscope level, the same syndrome of phenotypic expression as previously
described for homozygous t12 embryos (Smith, 1956).
A more recent study (Hillman, Hillman & Wileman, 1970) has shown, however, that the t12 homozygotes die over a range of preimplantation stages, death
not being limited to the late morula stage; that the t12lt12 genotype is a cell
lethal; and that the mutant embryos can be identified before developmental
arrest and degenerative changes by the presence of both nuclear lipid droplets
and nuclear fibrillo-granular bodies at each cleavage stage. In addition, t12/t12
embryos contain excessive cytoplasmic lipid droplets and, in the later cleavage
1
Authors'1 address: Department of Biology, Temple University, Philadelphia, Pennsylvania
19122, U.S.A.
43-2
686
N. HILLMAN AND R. HILLMAN
stages, frequently contain binucleate cells. These characteristics are visible only
at the ultrastructural level.
Since tw3Z has been described as being the same as t12, the present study has
been undertaken to determine if the tw32/tw32 genome elicited the same ultrastructural syndrome of phenotypic characteristics as described for the t12/t12
genome. Inasmuch as the ultrastructure of cleavage-stage wild-type embryos
and mutant embryos (t12jt12) have been previously reported (Hillman & Tasca,
1969; Hillman et al. 1970), those organelles which are ultrastructurally similar
in all embryos, at each cleavage stage, are not described.
MATERIALS AND METHODS
+ w32
Heterozygous (T /t ) animals were obtained from 8-week-old BALB/cT+
homozygous females mated to T\tw32 males. (The original T/tw32 breeding pairs
were obtained from Dr Dorothea Bennett.) Before mating inter se, the T+/tu'32
females from the parent cross were either superovulated [intraperitoneal injections of 10 i.u. of pregnant mare serum gonadotropin (PMS, Ayerst) followed
45 h later with 10 i.u. human chorionic gonadotropin (HCG, Organon)] or
timed-ovulated (2-5 i.u. PMS followed 45 h later with 2-5 i.u. HCG). The superovulated mice averaged 32 2-cell embryos per litter whereas the time-ovulated
mice averaged ten 2-cell embryos per litter. The superovulated females were
separated at random into two groups. Two-cell embryos were flushed from the
oviducts of females in the first group. Entire litters were separately placed into
culture and allowed to develop to the desired developmental stage (2-cell, 4-cell,
8-12 cell, early and late morulae, and substage 1 and 2 blastocysts) before being
processed for ultrastructural studies. From the second group of superovulated
females entire litters were removed from the oviducts or uteri at specific developmental stages and processed for electron microscopy immediately. Litters
from all timed ovulations were allowed to develop in vivo and were removed
from the mother at specific cleavage stages. BALBlcT+HBALB/cT+ embryos
developing either in vivo or in vitro served as controls.
The T+/tw32 male exhibits a high transmission ratio for the tw32 allele. Bennett
& Dunn (1964) have found the sperm transmission ratio of this allele to vary
between 0-94 and 0-74 depending upon the T+/tu32 tested. The averaged transmission ratio is 0-84 tw32:O-l6 T+. The pooled litters from large numbers of
T+/tw32 inter se matings should therefore contain approximately 40 % tw32 homozygotes. Our results (Table 1) correspond to those reported by Bennett and Dunn.
The expected ratios of tw32/tw32 embryos have been found in the pooled litters,
and the percentages of tw32 homozygous embryos obtained from either superovulated or timed-ovulated females do not differ significantly from those expected.
Superovulation does not, therefore, alter the transmission ratio of the female.
Entire litters of staged embryos, both those developing in vitro and those
developing in vivo, were fixed in 3 % glutaraldehyde in 0-1 M-PO 4 buffer (pH 7-4)
Studies o/t w32 /t vv32 embryos
687
Table 1. Stage of developmental arrest
T+ltw32 x T
Number
T+/T+ x T+/T+
V
Number
Total embryos
Arrested embryos
Stage of arrest
2-cell
8-cell
Early morula
Late morula
Blastocyst
1260
625
21
165
336
89
14
V
/o
/o
49-6
968
72
7-4
1-7
130
26-7
71
11
23
14
9
17
9
2-4
1-4
0-9
1-8
0-9
—
for 1 h, washed in 0-1 M-PO4 buffer overnight, postfixed in 1 % osmium tetroxide
(Millonig's, pH 7-3), dehydrated through a series of alcohols including absolute
alcohol and embedded in Epon. Thin sections were placed on uncoated copper
grids, stained with either lead citrate (Venable & Coggeshall, 1965), or lead
citrate preceded by 2 % uranyl acetate (Watson, 1958) and examined with
either a Zeiss 9 A or a Philips 300 electron microscope.
RESULTS
Seventy-two (7-4%) of the BALB\cT+\\BALB\cT+ embryos died during
development, the greatest number at the two-cell stage. Approximately 50 %
of the experimental embryos from heterozygous tw32 inter se matings died
(Table 1), mostly between the 8-cell and late morula stages. No significant
differences in either the time of lethality or percentage of embryos dying at
specific stages were noted between embryos developing in vitro and those developing in vivo. Because cell number alone is not a valid criterion for determining embryonic age during cleavage stages (particularly during the early and
late morula stages), both cell counts and ultrastructural examination have been
used to determine the time of death of the developmentally arrested experimental embryos. A major criterion for determining embryonic age is the ultrastructural appearance and shape of the nucleolus (Hillman & Tasca, 1969).
Based on nucleolar ultrastructure, the highest attrition of tw™ embryos occurs at
the 8-cell and early morula stages, with most dying as early morulae.
These developmentally arrested tlr32ltu™ embryos can be distinguished from
their developmentally arrested, phenotypically wild-type, litter-mates and
from correspondingly staged control embryos by additional ultrastructural
changes. These changes, either singly or in combination, distinguish the lethal
fM'32 homozygotes in 8-cell and older embryos. The increased attrition, therefore,
of embryos from /u'32 inter se matings in stages later than the 8-cell stage is a
function of the homozygous lethal genotype.
688
N. HILLMAN AND R. HILLMAN
Studies o/t w 3 2 /t w 3 2 embryos
689
w32
Five ultrastructural characteristics distinguish homozygous t
embryos
from their phenotypically normal litter-mates as well as from control embryos.
These characteristics are intranuclear lipid droplets, large clusters of cytoplasmic
lipid droplets, mitochondrial variants, binucleated cells and single cell lethality.
Both nuclear lipid droplets and excessive cytoplasmic lipid distinguish most of
the developmentally arrested older embryos obtained from T+/tw32 inter se
matings. In addition, 35-50 % of the viable 2-, 4- and 8-cell embryos obtained
from T+/tw32 inter se matings also contain nuclear and excessive cytoplasmic
lipid (Figs. 1, 2). Since the nuclear lipid droplets and large numbers of cytoplasmic lipid droplets are absent both in the remainder of the litter-mates and
in correspondingly staged control embryos, it is presumed that these structures
characterize the twS2/twS2 embryos at all cleavage stages. It has been noted that
both the nuclear lipid droplets and the cytoplasmic lipid droplets are found in
more cells and are more numerous as the embryos age (Fig. 3).
The mitochondrial variants are found only in 8-cell, early and late morula
?""32 embryos. Although these variant mitochondria are not found in all embryos
judged to be mutant because of their lipid inclusions, they do occur in a high
enough frequency to be considered characteristic of the homozygous phenotype.
In late 4-cell and 8-cell wild-type embryos, mitochondria are normally of two
forms. They are either round or ovoid, have arc-shaped cristae and contain an
electron-dense matrix; or they are elongate, have parallel cristae which traverse
the mitochondrion, and contain a less dense matrix. Only round, electron-dense
mitochondria are found in 2- and early 4-cell wild-type embryos (Hillman &
Tasca, 1969). A large number of the twS2 8-cell, early morulae and late morulae
embryos contain only rounded mitochondria with an electron-dense matrix
(cf. Figs. 4, 5). Additionally, significant numbers of the cells of early and late
morulae tw32 homozygotes also have mitochondria which contain crystalline
inclusions (Fig. 6a, b).
An additional characteristic of the twZ2ltw32 genotype is the presence of
binucleated cells. Again, these cells are not found in all embryos presumed to
be recessive homozygotes, but are found in a frequency high enough to be
considered characteristic of the twS2ltwZ2 genotype. These binucleate cells are
not found in mutant 2- and 4-cell embryos, but are found with increasing frequency in both viable and developmentally arrested older twS2 homozygotes.
Serial sections of these cells show that the two nuclei are completely separated
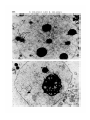
FIGURES 1 A N D 2
Fig. 1. A portion of a cell from an early 2-cell tK32/tu'32 embryo. Note the nuclear
lipid droplets (arrows), x 15000.
Fig. 2. A portion of a cell from an 8-cell r«32//"32 embryo. The nucleolus and
cytoplasmic organelles appear ultrastructurally normal for this developmental
stage. The nuclear lipid droplet (arrow) distinguishes this embryo as a homozygous
f"'32 embryo, x 13000.
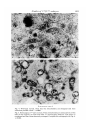
N. HILLMAN AND R. HILLMAN
»*i*5t*
Fig. 3. A developmentally arrested twZ2/tw32 morula. Note the presence of the large
lipid droplets in the cytoplasm. Similar cytoplasmic lipid droplets distinguish the
homozygous tw32 embryos before arrest and cellular degeneration, x 5200.
even though they are frequently juxtaposed. In the viable embryos, the
binucleated cell or cells which undergo karyokinesis but not cytokinesis remain
larger than the other blastomeres which continue to divide normally. In the
binucleate cells there are generally two nucleoli, rarely three nucleoli, per
nucleus. These nucleoli are ultrastructurally normal for the stage of development
attained by the cell (Fig. 7).
Although most twS2ltw*2 embryos die at the early morula stage, some stop
development as early as the 8-cell stage while some continue until the late
morula stage. When these embryos are examined as soon as they are found to be
arrested, not all the cells are in the same state of degeneration. They may contain cells undergoing mitoses as well as cells in an advanced degenerative stage.
Thus the tw32/tw32 genotype results in asynchronous cell death and can therefore
be considered a cell lethal.
With the exception of the phenotypic characteristics discussed above, the
cellular organelles observed in viable tw32 homozygotes do not differ from those
observed either in their phenotypically normal litter-mates or in their wild-type
counterparts. In all cases, the level of cellular ultrastructural differentiation is
dependent upon the stage of development attained by either the entire embryo
Studies o/t w 3 2 /t w 3 2 embryos
691
F I G U R E S 4 AND 5
Fig. 4. Wild-type morula. Note that the mitochondria are elongated and have
transverse parallel cristae. x 26000.
Fig. 5. Homozygous tu'32 early morula. These mitochondria are characteristic of the
cells of the majority of 8-cell and older f""32 homozygous embryos. The matrix is
condensed and the cristae aberrantly arranged. Compare this micrograph with Fig. 4.
x 26 000.
692
N. HILLMAN AND R. HILLMAN
Fig. 6. (a) Homozygous tw32 early morula. An additional distinguishing characteristic
of older twZ2/tu'*2 embryos is the presence of crystals (arrows) in the mitochondria. The
nucleus (N) of this cell appears to be breaking down, x 14000. (b) A higher magnification of two mitochondria containing crystals, from a tlc32/tu'32 early morula.
x 32000.
Fig. 7. A developmental^ arrested tw32/tK32 early morula. One of the cells is binucleate (nucleus: N). This cell and the other two cells are in advanced stages of
degeneration, x 3400.
Studies o/t w32 /t w32 embryos
693
or by its individual cells prior to developmental arrest. Differences in organelle
structure, other than those described above, become apparent only in advanced
stages of cellular degeneration. The stages of degeneration are the same as, and
occur in the same sequential order as those already reported for t12jt12 embryos
(Hillman et al. 1970). They are, therefore, not included in this report.
DISCUSSION
This paper is the second in a series describing the ultrastructural phenotype
of homozygous lethal tn\tn mice. The first in the series described the ultrastructural changes found in developmental^ arrested t12/t12 embryos (Hillman
et al. 1970). The homozygous t12/t12 and twS2ltwS2 genotypes are alike in that they
result in (1) preimplantation lethality, (2) cell lethality, (3) the presence of
nuclear lipid droplets and excessive cytoplasmic lipid, (4) the appearance of
binucleate cells, and (5) the same chronology of degenerative changes. Embryos
having the two homozygous mutant genotypes differ from each other in (1) their
lethal periods, (2) their nuclear inclusions, and (3) the presence or absence of
mitochondrial variants.
Both homozygotes can be distinguished from their litter-mates as early as the
2-cell stage by the presence of nuclear lipid droplets and excessive cytoplasmic
lipid. In addition, both homozygous mutant embryos often contain binucleate
cells, especially in the later cleavage stages. Usually there is only one binucleate
cell per embryo, but embryos of both genotypes may have as many as two or
three, either peripherally or centrally located. They remain larger than the other
cells, which continue to divide until individually arrested. The disproportionate
cell size results in aberrantly shaped embryos. Since the ?12//12 and tw32ltwS2
genotypes are characterized by individual cell death, developmentally arrested
embryos of both genotypes contain cells which are ultrastructurally normal as
well as cells which are in advanced stages of degeneration.
There are, as outlined above, certain phenotypic characteristics which distinguish the two homozygous mutant embryos from each other. It has been
found, for example, that the ?12/?12 genotype can result in embryonic lethality
as early as the 8-cell stage or as late as the early blastocyst stage. The majority of
/12//12 embryos, however, are arrested as late morulae (Hillman et al. 1970).
The present study shows that the twZ2ftwZ2 genotype also exhibits a range of
lethal periods. This range is from the 8-cell to the late morula stage, and unlike
tl2/t12 embryos, the highest percentage of tw32/tw32 attrition occurs at the early
morula stage. It should be emphasized that a distinction between early and late
morulae can only be achieved by an ultrastructural examination of mouse
embryos. This, in turn, probably accounts for the difference in the timing of the
lethal periods for twS2ltw*2 embryos as reported by Bennett & Dunn (1964) and
that found in the present study.
The additional morphological differences which distinguish tw32 homozygotes
694
N. HILLMAN AND R. HILLMAN
12
from t homozygotes can only be resolved at the ultrastructural level. For
example, the presence of small nuclear fibrillo-granular bodies distinguish t12/t12
embryos from their phenotypically normal litter-mates as early as the 2-cell
stage. These bodies are similar to nucleoli both in their structure and in their
response to enzymic digestion. They are unlike nucleoli in that they remain unlabelled in the presence of [3H]uridine (Hillman et al. 1970). Such nuclear bodies
are not found in twZ2 homozygotes and can therefore be used to distinguish the
two genotypes.
Finally, the mitochondria of t12/t12 embryos undergo the normal sequential
development which has been described for mouse cleavage-stage embryos
(Hillman & Tasca, 1969). Even in arrested t12/t12 embryos in advanced stages
of degeneration, the mitochondria appear normal for the developmental stage
attained by the embryo. These organelles maintain their structural integrity even in
those cells which have degenerated to the stage of cellular separation and vacuolization. Conversely, the mitochondria of some tw32/tw32 embryos differ from
those of their litter-mates as early as the 8-cell stage. In most mutant homozygotes,
the mitochondria do not undergo the normal structural transition which typifies
late 4- and early 8-cell mouse embryos. The continued presence of mitochondria
which are similar to the single mitochondrial form found in 2- and early 4-cell
wild-type embryos suggests that mitochondrial function, or cellular energy
metabolism, may differ between the homozygous mutant embryos and their
phenotypically and genetically wild-type counterparts. The fact that some
mitochondria of twZ2/twZ2 embryos contain crystalline inclusions supports this
hypothesis (Carafoli, Rossi & Lehninger, 1964; Greenawalt, Rossi & Lehninger,
1964; Lehninger, Carafoli & Rossi, 1967; Kotyk & Janacek, 1970; Trump,
Croker & Mergner, 1971; Bonucci, Derenzini & Marinozzi, 1973).
The two genotypes, therefore, elicit both similar and dissimilar phenotypic
expressions. Although the two alleles are each, in a homozygous condition,
preimplantation lethals, the evidence suggests that they are separate alleles, not a
recurrence of the same allele.
An ultimate method of testing the uniqueness of these two mutations is by
backcrossing each allele with a single isogenic stock until they are both on the
same isogenic background. Differences between the two could then be attributed
solely to the homozygosity of the allele and not to the effect of a modifying
genetic background. Such crosses have, however, generally led to either sterility
or fetal and postfetal inviability. At this time, therefore, the expression of the
two alleles must be examined on a heterogenic background. Under such conditions the alleles appear to be separate and distinct from each other. The ultrastructural characteristics suggest, however, that the primary effect of these two
alleles results in the same or in closely associated developmental aberrations.
The research was supported by U.S. Public Health Research Grant HD-00827. The authors
would like to acknowledge the technical assistance of Marie Morris and Geraldine Wileman.
Studies o/t w 3 2 /t w 3 2 embryos
695
REFERENCES
BENNETT, D. & DUNN, L. C. (1964). Repeated occurrences in the mouse of lethal alleles of
the same complementation group. Genetics 49, 949-958.
BONUCCI, E., DERENZINI, M. & MARINOZZI, V. (1973). The organic-inorganic relationship in
calcined mitochondria. /. Cell Biol. 59, 185-211.
CARAFOLI, E., ROSSI, C. S. & LEHNINGER, A. L. (1964). Cation and anion balance during
active accumulation of Ca ++ and Mg++ by isolated mitochondria. /. biol. Chem. 239,30553061.
CHESLEY, P. (1932). Lethal action in the short-tailed mutation in the house mouse. Proc.
Soc. exp. Biol. Med. 29, 437-438.
GREENAWALT, J. W., ROSSI, C. S. & LEHNINGER, A. L. (1964). Effect of active accumulation
of calcium and phosphate ions on the structure of rat liver mitochondria. /. Cell Biol. 23,
21-38.
HILLMAN, N., HILLMAN, R. & WILEMAN, G. (1970). Ultrastructural studies of cleavage stage
f12//12 mouse embryos. Am. J. Anat. 128, 311-340.
HILLMAN, N. & TASCA, R. (1969). Ultrastructural and autoradiographic studies of mouse
cleavage stages. Am. J. Anat. 126, 151-174.
KOTYK, A. & JANACEK, K. (1970). Cell Membrane Transport, Principles and Techniques. New
York: Plenum Press.
LEHNINGER, A. L., CARAFOLI, E. & Rossi, C. S. (1967). Energy-linked ion movements in
mitochondrial systems. In Advances in Enzymology (ed F. F. Nord), pp. 259-320. New
York: John Wiley & Sons.
SMITH, L. J. (1956). A morphological and histochemical investigation of a preimplantation
lethal (f12) in the house mouse. /. exp. Zool. 132, 51-83.
TRUMP, B. F., CROKER, B. P., JR. & MERGNER, W. J. (1971). The role of energy metabolism,
ion, and water shifts in the pathogenesis of cell injury. In Cell Membranes: Biological and
Pathological Aspects (ed. G. W. Richter & D. G. Scarpelli), pp. 84-128. Baltimore: The
Williams and Wilkins Company.
VENABLE, J. H. & COGGESHALL, R. (1965). A simplified lead citrate stain for use in electron
microscopy. /. Cell Biol. 25, 407-408.
WATSON, M. L. (1958). Staining of tissue sections for electron microscopy with heavy metals.
/. biophys. biochem. Cytol. 4, 475-478.
{Received 29 July 1974)