* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download lezione 3 bioluminescenza e proteine fluorescenti
Survey
Document related concepts
Cell nucleus wikipedia , lookup
Cell growth wikipedia , lookup
Endomembrane system wikipedia , lookup
Cytokinesis wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Signal transduction wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell encapsulation wikipedia , lookup
Tissue engineering wikipedia , lookup
List of types of proteins wikipedia , lookup
Transcript
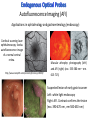
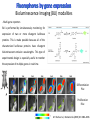
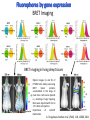
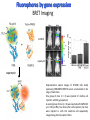
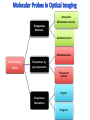
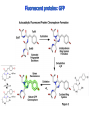
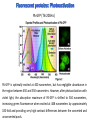
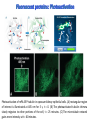
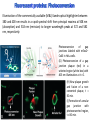
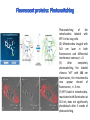
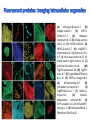
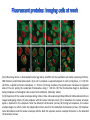
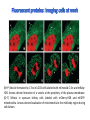
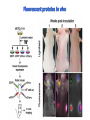
Absorption Multiphoton emission Endogeneous Molecules Autofluorescence Bioluminescence Optical Imaging Fluorophores by Probes gene expression Fluorescent proteins Organic Exogeneous fluorophores Inorganic Absorption Absorption of light in the NIR window is mainly due to oxy/deoxy haemoglobin and myoglobin. DOT contrast is primarily generated by absorption Tissue components with specific structural properties can emit harmonics waves under NIR illumination, whose frequency is multiple of the exciting photons (e.g. SHG, THG). Applications in intravital microscopy f: fat cells -‐ m: myofiber -‐ n: nerve -‐ c: collagen Autofluorescence Tissues contain low amounts of fluorescent molecules Autofluorescence can be exploited for diagnostic purposes Autofluorescence Imaging (AFI) Applications in ophtalmology and gastroenterology (endoscopy) Confocal scanning laser ophthalmoscopy fundus autofluorescence image of a normal central retina. http://www.revophth.com/content/d/retina/c/22655/ Macular athrophy: photography (left) and AFI (right) (exc. 535-‐580 nm – em. 615-‐715) Suspected lesion of early gastric cancer Left: white light endoscopy Right: AFI. Contrast confirms the lesion (exc. 390-‐470 nm , em 500-‐630 nm) Bioluminescence Imaging (BLI) Main Pro: Reduced tissue background due the lack of endogenous bioluminescent reactions in mammalian tissue λem 562 nm – quantum yield 0.4 Sensitivity: 240000 molecules Substrate: D-‐luciferin λem 480 nm (red shifted version available) quantum yield 0.07 Substrate: coelenterazine Does not require exogenous substrate λem 490 nm – low quantum yield (now improved) Bioluminescence Imaging (BLI) In vivo comparison between Fluc (Firefly Luciferase) and Lux (bacterial luciferase) BLI systems (subcutaneous injection) A) FLuc-‐tagged cells and luciferin B) Lux-‐tagged cells alone Very different emission intensity, but almost no background. For Lux a longer “exposition” time (60 sec vs 1 sec) was used DM Close et al., J. Biomed Opt (2011) 16:047003 Bioluminescence Imaging (BLI) modalities -‐ Steady State Bioluminescently tagged cells are imaged over time to determine if light output is increasing or decreasing compared to the initial state. Commonly, bioluminescent cells are injected into an animal model to determine the kinetics of tumorigenesis and growth, and anticancer drug efficacy as well. High sensitivity detection of BLI-‐tagged tumour cells (subcutaneous injection) Bioluminescence Imaging (BLI) modalities -‐ Multi-‐gene reporters BLI is performed by simultaneously monitoring for expression of two or more divergent luciferase proteins. This is made possible because all of the characterized luciferase proteins have divergent bioluminescent emission wavelengths. This type of experimental design is especially useful to monitor the expression of multiple genes in real time. Differentiation Pluc Proliferation Rluc M. Vilalta et al., Biomaterials (2009) 30: 4986–4995 Bioluminescence Imaging (BLI) modalities -‐ Multi-‐components Imaging modality where bioluminescence is exploited to promote photoluminescence from an associated fluorescent reporter protein, thus avoiding the stimulation by an exogenous light source. This process is known as Bioluminescence Resonance Energy Transfer (BRET). It can be used to boost the luminescent signal of a bioluminescent reporter, or to determine the interaction of two components of interest within a given system. RET mechanism requires that donors/acceptors are close in space (< 10 nm) A widely known example of the utility of BRET is to demonstrate the presence of G protein coupled receptor dimers on the surface of living cells. By tagging a subset of β2-‐adrenergic receptor proteins with RLuc and a subset with the red-‐shifted variant of green fluorescent protein, YFP, it was possible to detect both a bioluminescent and fluorescent signal in cells expressing both variants, but no fluorescent signal in cells expressing only YFP HEK293 living cells BRET Ratio: YFP/Rluc S. Angers, PNAS, 97, 3684, 2000 BRET Imaging BRET imaging in living deep tissues Optical images (A and B) of HT1080 cells stably expressing BRET fusion proteins accumulated in the lungs of nude mice. Cells were injected i.v., resulting in lungs trapping. Mice were injected with CLZ at 1.5 h after cell injection. Importance of red-‐shift illumination. A. Dragulescu-‐Andrasi et al. ,PNAS, 118, 12060, 2011 BRET Imaging control rapamycin rapamycin Representative optical images of HT1080 cells stably expressing FRB-‐FKBP12 BRET6 sensor accumulated in the lungs of nude mice. One group of mice (n = 8) was injected 2 h before cell injection with 40 μg rapamycin A second group of mice (n = 8) was injected with DMSO (20 μL in 130 μL PBS). Two hours after cells injection, the mice were injected i.v. with CLZ substrate and sequentially imaged using donor/acceptor filters. Bioluminescence Imaging (BLI) Substrate administration (D-‐luciferin or coelenterazine) -‐ Intraperitoneal Excellent absorption for gastrointestinal tract, pancreas, and spleen, but low reproducibility -‐ Intravenous Whole-‐body diffusion with similar rate. Low doses required. Shorter emission time -‐ Subcutaneous Good reproducibility, less damage at the injection site (important for repeated administration) Bioluminescence Imaging (BLI): Applications Firefly/Renilla Bacterial Absorption Multiphoton emission Endogeneous Molecules Autofluorescence Bioluminescence Optical Imaging Fluorophores by Probes gene expression Fluorescent proteins Organic Exogeneous fluorophores Inorganic Green-‐FP was the first fluorescent protein discovered in 70’s. It was isolated from jellyfish where the fluorescence was stimulated by an energy transfer from the luciferase aequorin. In 1992, a fully-‐length clone encoding Aequorea GFP was prepared. Since then, GFP was expressed in many cells of different living organisms, thereby revolutionizing cell/molecular biology. The GFP chromophore is encoded by the primary amino acid sequence, (ca. 240 aa) and it forms spontaneously without the requirement for cofactors or external enzyme components, through a self-‐catalyzed protein folding mechanism and intramolecular rearrangement. The genetically encoded GFP provided for the first time the ability to label specific proteins inside the living cell without the need for exogenous synthetic or antibody-‐ labeled fluorescent tags. The GFP chromophore derives from the cyclization of the sequence “Ser65-‐Tyr66-‐ Gly67” that occurs in presence of oxygen, and it is catalyzed by Arg96 and Glu222 The chromophore is protected => high photostabilty and quantum yield N-‐ and C-‐termini can be used to give fusion proteins without affecting fluorophore properties The fluorescence properties of GFP are very dependent on the 3D structure of the amino acid surrounding chromophore. residues the Mutations that alter both the residues immediately adjacent to the chromophore (but also far regions) generally have a profound impact on the spectral properties of the protein. Wild type GFP EGFP S65T mutant -‐ 65Ser =>Thr 5 times brighter, faster maturation time, readily detectable with common filters Tyr66His Tyr66Trp Ser65Thr Thr203Tyr The most red-‐shifted Aequorea FPs are YFPs Red FPs was obtained from Anthozoa sp. Many modifications were performed to shorten maturation times, improve brightness, and favor monomeric forms. This research led to “mFruits” series (mBanana, mOrange, mCherry, mStrawberry, mPlum) Aequorea wild type GFP mCherry HeLa cell mCerulean => nucleus mKusabira Orange => peroxisome mPlum => mitochondria Sapphire mutation (Tyr203Ile) dramatically increases Stokes shift The photophysical properties of fluorescent proteins are often extremely complex and can involve several distinct emissive and non-‐emissive (dark) states, as well as on-‐and-‐off blinking behavior when observed at the single molecule level. Ø Photoactivation Fluorophore is activated from very low level to bright emission upon illumination with ultraviolet or violet light. Ø Photoconversion The emission is optically converted from one bandwidth to another. Ø Photoswitching The emission can alternatively be turned “on” or “off” with specific illumination PA-‐GFP (Thr203His) PA-‐GFP is optimally excited at 400 nanometers, but has negligible absorbance in the region between 450 and 550 nanometers. However, after photoactivation with violet light, the absorption maximum of PA-‐GFP is shifted to 504 nanometers, increasing green fluorescence when excited at 488 nanometers by approximately 100-‐fold and providing very high contrast differences between the converted and unconverted pools. Photoactivation of mPA-‐GFP-‐tubulin in opossum kidney epithelial cells. (A) rectangular region of interest is illuminated at 405 nm for 5 s, t = 0. (B) The photoactivated tubulin chimera slowly migrates to other portions of the cell, t = 25 minutes. (C) The microtubule network gains more intensity at t = 60 minutes. Illumination of the commercially available (MBL) Kaede optical highlighter between 380 and 400 nm results in a rapid spectral shift from principal maxima at 508 nm (absorption) and 518 nm (emission) to longer wavelength peaks at 572 and 582 nm, respectively. Photoconversion of gap junctions labeled with mEos2– Cx43 in HeLa cells. (G) Photoconversion of a gap junction plaque (red) in a selected region (white box) with 405 nm illumination at t = 0. (H) New plaque growth and fusion of a non-‐ converted plaque, t = 50 min. (I) Formation of annular gap junction with photoconverted region, t = 80 min. Photoswitching of the mitochondria labeled with KFP1 in fox lung cells. (D) Mitochondria imaged with 543 nm laser in both fluorescence and differential interference contrast, t = 0. (E) After completely photoswitching the labeled chimera “off” with 488 nm illumination, the mitochondria now appear devoid of fluorescence, t = 3 min. (F) KFP1 label in mitochondria, reactivated with illumination at 543 nm, does not significantly photobleach after 5 rounds of photoswitching. (A) mOrange2-‐β-‐actin-‐C-‐7. (B) mApple-‐Cx43-‐N-‐7. (C) mTFP1-‐ fibrillarin-‐C-‐7. (D) mWasabi-‐ cytokeratin-‐N-‐17. (E) mRuby-‐annexin (A4)-‐C-‐12. (F) mEGFP-‐H2B-‐N-‐6. (G) EBFP2-‐β-‐actin-‐C-‐7. (H) mTagRFP-‐T-‐ mitochondria-‐N-‐7. (I) mCherry-‐C-‐Src-‐ N-‐7. (J) mCerulean-‐paxillin-‐N-‐22. (K) mKate-‐clathrin (light chain)-‐C-‐15. (L) mCitrine-‐VE-‐cadherin-‐N-‐10. (M) TagCFP-‐lysosomes-‐C-‐20. (N) TagRFP-‐ zyxin-‐N-‐7. (O) superfolderGFP-‐lamin B1-‐C-‐10. (P) EGFP-‐α-‐v-‐integrin-‐N-‐9. (Q) tdTomato-‐Golgi-‐N-‐7. (R) mStrawberry-‐vimentin-‐N-‐7. (S) TagBFP-‐Rab-‐11a-‐C-‐7. (T) mKO2-‐LC-‐ myosin-‐N-‐7. (U) DsRed2-‐ endoplasmic reticulum-‐N-‐5. (V) ECFP-‐α-‐tubulin-‐C-‐6. (W) tdTurboRFP-‐ farnesyl-‐ C-‐5. (X) mEmerald-‐EB3-‐N-‐7. (Y) mPlum-‐CENP-‐B-‐N-‐22. (A–D) Observing mitosis in dual-‐labeled normal pig kidney (LLC-‐PK1 cell line) epithelial cells stably expressing mCherry-‐ H2B (histones) and mEmerald-‐α-‐tubulin. (A) A cell in prophase is captured adjacent to cells in interphase, t = 0. (B) The cell forms a spindle and enters metaphase, t = 20 min. (C) During anaphase, the spindle poles translocate to opposite sides of the cell, pulling the condensed chromosomes along, t = 60 min. (D) The chromosomes begin to decondense during telophase as the daughter cells recover from cell division (mid-‐body visible). (E–H) Dispersion of the nuclear envelope during mitosis. HeLa cells expressing mRuby-‐H2B and mEmerald-‐lamin-‐B1 are imaged undergoing mitosis. (E) Late prophase with the nuclear envelope intact. (F) In metaphase, the nuclear envelope signal is dispersed in the cytoplasm. Note the detached chromosome (arrow). (G) During late anaphase, the nuclear envelope begins to reform. Note the independent mitosis event for the detached chromosome (arrow). (H) Telophase nuclei decondense and the nuclear envelope reforms. Note the separate nuclear envelope formation on the detached chromosomes (arrow). (M–P) Vesicle formation by C-‐Src in U2OS cells labeled with mEmerald-‐C-‐Src and mRuby-‐ H2B. Arrows denote formation of a vesicle at the periphery of the plasma membrane. (Q–T) Mitosis in opossum kidney cells labeled with mCherry-‐H2B and mEGFP-‐ mitochondria. Arrows denote localization of mitochondria to the mid-‐body region during cell division http://zeiss.magnet.fsu.edu/articles/probes/index.html