* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Phosphatidylinositol 4-Phosphate Formation at ER Exit Sites
Survey
Document related concepts
Hedgehog signaling pathway wikipedia , lookup
P-type ATPase wikipedia , lookup
Cell growth wikipedia , lookup
Lipid bilayer wikipedia , lookup
Cell culture wikipedia , lookup
Magnesium transporter wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Cell encapsulation wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
SNARE (protein) wikipedia , lookup
Cytokinesis wikipedia , lookup
Signal transduction wikipedia , lookup
Cell membrane wikipedia , lookup
Lipid signaling wikipedia , lookup
Transcript
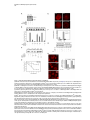
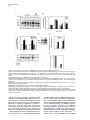
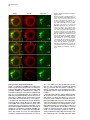
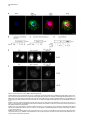
Developmental Cell 11, 671–682, November, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.devcel.2006.09.001 Phosphatidylinositol 4-Phosphate Formation at ER Exit Sites Regulates ER Export Anna Blumental-Perry,1 Charles J. Haney,1 Kelly M. Weixel,1,2 Simon C. Watkins,1 Ora A. Weisz,1,2 and Meir Aridor1,* 1 Departments of Cell Biology and Physiology 2 Renal-Electrolyte Division University of Pittsburgh School of Medicine 3500 Terrace Street Pittsburgh, Pennsylvania 15261 The mechanisms that regulate endoplasmic reticulum (ER) exit-site (ERES) assembly and COPII-mediated ER export are currently unknown. We analyzed the role of phosphatidylinositols (PtdIns) in regulating ER export. Utilizing pleckstrin homology domains and a PtdIns phosphatase to specifically sequester or reduce phosphorylated PtdIns levels, we found that PtdIns 4-phosphate (PtsIns4P) is required to promote COPII-mediated ER export. Biochemical and morphological in vitro analysis revealed dynamic and localized PtsIns4P formation at ERES. PtdIns4P was utilized to support Sar1-induced proliferation and constriction of ERES membranes. PtdIns4P also assisted in Sar1-induced COPII nucleation at ERES. Therefore, localized dynamic remodeling of PtdIns marks ERES membranes to regulate COPII-mediated ER export. capture by the COPII coat (Aridor et al., 1998, 2001; Bannykh et al., 1998; Barlowe, 2003; Kuehn et al., 1998). Nevertheless, the affinities of coat-cargo interactions are relatively low and may not support cargo recognition by themselves (Miller et al., 2003; Mossessova et al., 2003). Regulated enhancement of the binding avidity between cargo and the coat at ERES may provide a plausible mechanism to control ER export. Thus targeted assembly of COPII at defined ERES where cargo is concentrated provides a mechanism to regulate COPIImediated cargo export from the ER. How are ERES maintained as unique domains within the fluid ER membranes? Lipid composition may regulate protein machinery needed to maintain ERES organization. Thus, identifying specific lipid signals that dynamically mark ERES may provide key insight into ERES function. Distinct phosphatidylinositols (PtdIns) are known to function as specific regulators of protein sorting and traffic in the late secretory and endocytic pathways (De Matteis and Godi, 2004; Simonsen et al., 2001; Wenk and De Camilli, 2004). Our previous studies suggest a functional role for phospholipase D (PLD) activity in ER export (Pathre et al., 2003). Phosphorylation of PtdIns is enhanced by PLD activity and can generate specific lipid signals. Kinase (possibly lipid kinase) activity is required to regulate ER export (Aridor and Balch, 2000). We therefore hypothesized that spatially regulated phosphorylation of PtdIns supports ERES assembly and function. Introduction Results The endoplasmic reticulum (ER) is a polarized compartment. Cotranslational insertion of newly synthesized polypeptides takes place in the ribosome-bound rough ER, whereas protein selection for export occurs from ribosome-free smooth membranes (Palade, 1975). Membrane protrusions are formed on smooth ER membranes at ER exit sites (ERES) where cargo proteins destined for export are concentrated (Bannykh et al., 1996). ERES are distributed at the cell periphery and perinuclear region (Aridor et al., 2004; Bannykh et al., 1996; Bevis et al., 2002; Stephens, 2003). Vesicles and elongated saccular/tubular carriers that protrude from ERES export cargo proteins to the Golgi complex (Bannykh et al., 1996; Mironov et al., 2003). Cargo proteins are selected for export at ERES by the activity of the cytosolic COPII coat, composed of the small GTPase Sar1 and the Sec23/24 and Sec13/31 protein complexes (Antonny and Schekman, 2001). COPII proteins are sequentially recruited to ERES following the activation of Sar1 (Figure 1A) (Aridor et al., 1998; Matsuoka et al., 1998). The recruited Sec23/24 complex exposes extensive protein surfaces that present multiple peptide-binding pockets (Miller et al., 2003; Mossessova et al., 2003). These binding sites specifically recognize a variety of ER export motifs on cargo proteins to mediate their PtdIns Are Required to Support COPII-Mediated ER Export Specific PtdIns may serve as membrane codes to support ER export. Thus, we sequestered PtdIns formed on ER membranes to test their role in vesicle formation from the ER. We utilized an in vitro vesicle-budding assay to follow the release of the biosynthetic cargo reporter tsO45 VSV-G from ER. tsO45 VSV-G (referred to hereafter as VSV-Gts) is a temperature-sensitive protein that displays a reversible folding defect. It is arrested in the ER at a nonpermissive temperature (39.5 C) yet synchronously folds trimerizes and exits the ER upon shift to a permissive temperature (32 C) (Doms et al., 1987). Microsomes expressing VSV-Gts were incubated with cytosol in the presence or absence of the polybasic antibiotic neomycin. At the end of incubation, the vesicle fraction was separated from donor membranes by centrifugation, and the mobilization of VSV-Gts to vesicles was determined by western blotting (Figure 1A). Neomycin, which binds and sequesters phosphorylated PtdIns, inhibited vesicular export of VSV-Gts from the ER (Figure 1B). However, neomycin has additional targets that can affect ER export. Therefore, to examine the contributions of specific PtdIns to ER export, recombinant GST-tagged pleckstrin homology (PH) domains with high binding affinity for specific PtdIns were added to the assay (Lemmon and Ferguson, 2001). Importantly, Summary *Correspondence: [email protected] Developmental Cell 672 Figure 1. The Fapp1-PH Domain Inhibits ER Export (A) Schematic representation of COPII coat assembly and ER export. The release of cargo proteins to the vesicular fraction was measured by differential centrifugation. M designates medium-speed donor-membranecontaining pellet (16,000 3 g). H designates high-speed vesicle containing pellet (186,000 3 g). (B–D) VSV-Gts-containing microsome membranes were incubated with cytosol for 30 min at 32 C in the presence or absence of neomycin (1.5 mM) (B) or the indicated GST-tagged PH domains (C and D), and the release of VSV-Gts (B and C) or Bet1 (D) to the vesicle fraction was analyzed. (E–G) VSV-Gts-expressing microsomes were incubated with cytosol (E) or purified COPII components (Sar1 [1 mg], Sec23/24 [0.4 mg], and Sec13/31 [6 mg], 40 ml final volume) (F and G) in the presence of PLCd1-PH (E and F) or Fapp1-PH (G) as indicated for 30 min at 32 C. The release of VSV-Gts to the vesicular fraction was analyzed. The results are representative of at least two independent experiments. GST-PH domains were tested at concentrations within the range of their binding affinities to PtdIns containing liposomes. GST-Fapp1-PH, which selectively interacts with PtdIns4P (Dowler et al., 2000), inhibited VSV-Gts export at concentrations of 2–3 mM (Figures 1C and 1G and titration in Figure 2D). GST-Fapp1-PH also inhibited the mobilization of COPII vesicle SNARE protein Bet1 (Figure 1D), suggesting that sequestration of PtdIns4P led to general inhibition of ER export. In contrast, addition of GST, or GST fused to the PH domains of dynamin 2, which has weak affinities for PtdIns(4,5)P2 and PtdIns(3,4,5)P3 (Lee and Lemmon, 2001), or Grp1, which has high affinity for PtdIns(3,4,5)P3, did not modulate VSV-Gts export from the ER (Figure 1C). The PH domain of PLCd1, which binds PtdIns(4,5)P2 (Lemmon and Ferguson, 2001), a possible product of PtdIns4P phosphorylation, inhibited ER export to a variable degree at higher concentrations (7–15 mM) (Figure 1E). Direct analysis of PtdIns phosphorylation suggested that kinase activities in cytosol promoted PtdInsP2 synthesis (see below, Figure 3F), yet ER export was effectively reconstituted in the absence of cytosol with purified mammalian COPII components (Aridor et al., 1998). Indeed, PLCd1-PH was ineffective in inhibiting the budding reaction reconstituted without cytosol in the presence of purified COPII proteins (Figure 1F). In contrast, full inhibition of vesicle formation by GST-Fapp1-PH domain was observed at concentrations as low as 2 mM, when budding was reconstituted with purified COPII (Figure 1G). These results suggest that PtdIns4P may function as a possible regulator of ER export. The Inhibition of ER Export by Fapp1-PH Is Mediated by PtdIns4P Binding Fapp1 proteins localize to the TGN in NRK cells. TGN localization is mediated by the Fapp1-PH domain and is directed by specific interactions of the domain with PtdIns4P lipids and ARF1 (Godi et al., 2004). We have previously demonstrated that under the budding assay conditions utilized, inhibition of ARF1 function by the addition of high concentrations of dominant-negative myrARF1(T31N) or by depletion of cytosolic COPI does not affect the reconstituted COPII-dependent vesicle formation step (Rowe et al., 1996). Indeed, ARF1 is not present in the vesicle formation assay reconstituted with purified COPII coat components under conditions where full inhibition of ER export by Fapp1-PH is observed (Figure 2A). We did not observe specific interactions between Sar1 and any of the PH domains tested (not shown). We thus analyzed whether the inhibitory effect of Fapp1-PH on ER export is due to PtdIns4P binding. Fapp1-PH added together with PtdIns4Pcontaining liposomes (but not PtdIns-free liposomes) displayed little inhibition of ER export (Figure 2B). The added liposomes may have augmented membrane PtdIns4P content or simply sequestered Fapp1-PH; in either case PtdIns4P binding was required for Fapp1PH function. We further mutated the PtdIns4P binding site of Fapp1-PH, by replacing a conserved tryptophan and arginine residues with asparagine and lysine (W15N, R18K, mutated protein termed Fapp1-PHNK) akin to the mutations that inhibit the recognition of PtdIns(4,5)P2 by PLCd1-PH (Figure S1A; see the Supplemental Data available with this article online). Fapp1PHNK displayed significantly reduced affinity for PtdIns4P as analyzed in protein-lipid overlay and liposome binding assays (Figures S1B–S1E). Consistent with its reduced affinity for PtdIns4P, Fapp1-PHNK failed to affect vesicle formation at concentrations of up to 5 mM (Figures 2C–2D). To further explore a possible role for PtdIns4P in ER export, the synchronized mobilization of VSV-Gts from the ER was reconstituted in vitro in semi intact cells and measured by indirect immunofluorescence (IF) (Plutner et al., 1992). VSVts-infected NRK cells were permeabilized and incubated in the presence of WT or mutated Fapp1-PH domains. Addition of rat liver cytosol (RLC) quantitatively mobilized VSV-Gts from the reticular ER to punctate vesicular tubular Regulation of ER Export by Phosphoinositides 673 Figure 2. PtdIns4P Binding Mediates Fapp1-PH Inhibition of ER Export (A) VSV-Gts-infected microsome membranes were incubated with purified COPII components (as in Figure 1) in the presence of GST-Fapp1-PH domain as indicated for 30 min at 32 C. The mobilization of VSV-Gts to the vesicular fraction and the presence of ARF1 in the analyzed fractions were determined by western blot. Recombinant ARF1 was loaded separately as a control. (B) VSV-Gts-containing microsomes were incubated with cytosol in the presence of the indicated concentrations of GST-Fapp1-PH domain with or without addition of liposomes (Lpsm) composed of PC or PC/PtdIns4P (420/50 mM) for 30 min at 32 C as indicated. The release of VSV-Gts to the vesicular fraction is quantified in the graph below. Data are averaged from three independent experiments (means 6 SD). (C) VSV-Gts-expressing microsomes were incubated with cytosol in the presence or absence of GST-Fapp1-PHWT or GST-Fapp1-PHNK for 30 min at 32 C, and the budding of VSV-Gts was determined. (D) Quantitation of VSV-Gts released into vesicular fractions in the presence of varying concentrations of GST-Fapp1-PHWT and Fapp1-PHNK domains. Data are means 6 SD derived from five independent experiments. (E) VSVts-infected cells were permeabilized and incubated in the presence or absence of rat liver cytosol (RLC) GST-Fapp1-PHWT (3 mM) or GSTFapp1-PHNK (5 mM) as indicated for 30 min at 32 C. The mobilization of VSV-Gts from reticular ER (upper left image) to VTCs (upper right image) was determined by IF. The images are a projection of five consecutive 0.1 mm optical sections. Scale bar is 5 mm. (F) Liposomes (40 ml) composed of phosphatidylserine (500 mM) and PtdIns4P (100 mM) were incubated at 32 C for 3 min with indicated amounts of the cytosolic domain (amino acid 1–507) of Sac or SacC392S mutant. Reactions were terminated by the addition of NEM (50 mM). Phosphate release was quantified by a malachite green assay. Data are means 6 SD of triplicate samples. (G) VSV-Gts microsomes (40 ml final volume) were incubated with cytosol in the presence or absence of of Sac1 (0.9 mg) or Sac1C392S (3.6 mg) as indicated for 30 min at 32 C and the mobilization of VSV-Gts to the vesicle fraction was determined. The results are representative of at least three independent experiments. (H) VSVts infected cells were permeabilized and incubated (220 ml final volume) in the presence or absence of RLC and Sac1 (1 mg) as indicated for 30 min at 32 C. The movement of VSV-Gts from reticular ER (upper image) to VTCs (middle image) and the effects of Sac1 on VSV-Gts mobilization (lower image) were determined by IF. Quantitation of VSV-Gts transport to VTCs at 20 and 30 min incubations in the presence or absence of the indicated amounts of Sac1 is shown on the right panel. The number of VSV-Gts containing VTCs was derived from analysis of random fields of cells by automatically counting 100 cells or more for each data point. Developmental Cell 674 Figure 3. Sar1 Recruitment and Activation on ER Membranes Stimulates PtdInsP and PtdInsP2 Formation (A) ER enriched membrane fractions were incubated with buffer (control) or with Sar1-GTP (H79G) (2 mg, 60 ml final volume) for 15 min at 32 C. Membranes were washed and incubated in the presence of g-32P-ATP and cytosol for the indicated times at 32 C. At the end of incubations, lipids were extracted and separated by TLC. Phospholipids were quantified on a phosphor imager, and lipid markers were identified by iodine vapors. (B) Quantitation of (A) (means 6 SEM derived from four independent experiments). (C) ER enriched membrane fractions, were incubated with buffer (control), Sar1-GTP, or Sar1-GDP (T39N, 2 mg of each, 60 ml final volume) for 15 min at 32 C. Membranes were washed and further incubated for 3 min as described in (A). Data are means 6 SD of three independent experiments. (D) Membranes were incubated and lipids extracted as described in (C) except that orthovanadate (200 mM) was included in the second incubation to inhibit phosphatase activities. (E) Quantitation of (D). Data represent means 6 SD of four independent experiments. (F) Membranes were incubated in the absence or presence of Sar1-GTP (6 mg, 40 ml final volume) with g-32P-ATP in the presence or absence of wortmannin (5 mM) or adenosine (500 mM) for 9 min at 32 C as indicated. Lipids were extracted and processed as described above. (G) The effect of indicated inhibitors (analyzed as in [F]) was calculated by dividing the fold stimulation of PtdInsP formation by Sar1 (over control) in the presence or absence of inhibitors, at time points at which peak PtdInsP formation was observed. Data are mean 6 SD of three to five independent experiments. clusters (VTCs) (Figure 2E, upper right panel). VSV-Gts mobilization was markedly inhibited by neomycin (not shown) and by the addition of GST-Fapp1-PH domain (Figure 2E, bottom left panel). In contrast, GST-Fapp1PHNK did not inhibit the mobilization of VSV-Gts to VTCs (Figure 2E, bottom right panel). Together, the structure-function analysis and liposome rescue experiments suggest that the inhibitory effect of Fapp1-PH on ER export is probably due to sequestration of PtdIns4P. We tested whether reduction of PtdIns4P levels by the yeast phosphatase Sac1 can modulate ER export. Sac1 is an ER and Golgi-localized enzyme that dephosphorylates the 3 and 4 positions of PtdIns3P or PtdIns4P, exerts low phosphatase activity toward PtdIns(3,5)P2, does not act on PtdIns (4,5)P2, and exhibits in vivo preference for PtdIns4P (Hughes et al., 2000). We produced the cytosolic domain of Sac1 (amino acid 1–507) and tested its phosphatase activity by using a colorimetric assay (Maehama et al., 2000). Sac1 efficiently dephosphorylated PtdIns4P present in PtdIns4P-containing liposomes, while a catalytic inactive mutant (Sac1C392S) did not (Figure 2F). Sac1 inhibited vesicle budding Regulation of ER Export by Phosphoinositides 675 from the ER, whereas the catalytically inactive mutant (Sac1C392S) had no effect (Figure 2G). The inhibitory effect was reproduced in morphological ER export assays. Sac1 quantitatively delayed the cytosol-dependent mobilization of VSV-Gts to VTCs as analyzed by IF in permeabilized VSV-Gts expressing cells (Figure 2H). Importantly, Sac1 mediated delay of VSV-Gts mobilization to VTCs correlated with the inhibition detected in budding assays (Figure 2G) to further support a role for PtdIns4P in regulating ER export. Sar1 Recruitment to ER Membranes Enhances the Formation of PtdInsP and PtdInsP2 Sar1 recruitment to ER membranes was unaffected by the sequestration of PtdIns4P (not shown). Therefore, we analyzed whether Sar1 recruitment can affect PtdIns4P levels. Sar1 binding and activation is specific for ER membranes (Figure 6A). We nevertheless fractionated rat liver to enrich for ERES (Figure S2) and reduce the signal derived from lipid kinase activities present in crude membrane preparations. A two-stage assay was utilized to analyze the phosphorylation state of PtdIns in response to Sar1 activation (Figure 3). In stage 1, membranes were incubated in the absence or presence of GTPase-deficient Sar1 (Sar1-GTPH79G). The membranes were washed and incubated (second stage) in the presence of g-32P-ATP and cytosol. Subsequently, lipids were extracted and separated by thinlayer chromatography (TLC), and phosphorylated PtdIns visualized by autoradiography. Recruitment of Sar1-GTP to membranes caused a cyclic increase in PtdIns mono- and bis-phosphate levels, with a maximal (2-fold) effect observed at 3 min (Figures 3A, 3B, and 3C) and subsequent increases at 9 min. This activity is similar to the previously reported transient elevation in phosphorylated PtdIns observed upon incubation of Golgi membranes with activated ARF1 (Godi et al., 1999; not shown) and may reflect limiting PtdIns substrate levels that are dynamically regenerated on the membranes. Activated Sar1 also increased 32P-phosphatidic acid (PA) levels generated by diacyl glycerol (DAG) kinase activity (Figure 3B). The elevation in PtdInsP and PtdInsP2 levels required Sar1 activation as incubations with Sar1-GDPT39N, a Sar1 mutant deficient in GTP binding that cannot support ER export, did not enhance PtdIns phosphorylation (Figure 3C) (Aridor et al., 1995). Sar1 could increase PtdIns levels through enhanced PtdIns phosphorylation or through inhibition of PtdIns phosphatase activities. To discriminate between these possibilities, we analyzed PtdIns phosphorylation in the presence of the phosphatase inhibitor orthovanadate. In the presence of orthovandate, incubations with Sar1-GTP led to a 4-fold increase in PtdInsP and PtdInsP2 levels over control, suggesting that kinase activities were enhanced under these conditions (Figures 3D–3E). Sar1 also stimulated PtdInsP formation when measured in one-stage incubations carried out without cytosol in the presence g-32P-ATP (up to 11-fold stimulation observed). Under these conditions, formation of PtdInsP2 was largely diminished (Figure 3F). Therefore, in agreement with our analysis of ER export (Figure 1G), Sar1-regulated kinase activity that generates PtdInsP is membrane associated. Pharmacological analysis indicated that unlike the PtdIns kinases (PI Kinases) activated by ARF1 (Godi et al., 1999), Sar1-induced PtdInsP formation was insensitive to wortmannin, a drug that inhibits PI 3-kinases and type III PI 4-kinases (Figure 3F), suggesting that these proteins are not involved in the regulating ER export. In agreement, Sar1-induced phosphorylation of PtdIns was inhibited by adenosine, added at equimolar concentration to ATP (Figures 3F and 3G). This inhibition is characteristic of type II PI 4-kinases, which display comparable affinities to ATP and adenosine (Balla et al., 2002). Therefore Sar1 activates a membrane-associated type II PI 4-kinase. The stimulation of PtdInsP formation by Sar1 supports the role we uncovered for PtdIns4P on ER membranes (Figures 1 and 2). Sar1 activation may provide coupling between COPII assembly and PtdIns4P formation during ER export. Dynamic and Localized Formation of PtdIns4P at ERES To provide a unique environment within fluid ER membranes, lipid remodeling should be dynamic. Because Sar1 activation stimulated only limited PtdInsP, PtdInsP2, or PA production (Pathre et al., 2003), lipid remodeling should also be localized to ERES in order to be effective. To test these predictions, we reconstituted the synchronized export of VSV-Gts from the ER in permeabilized VSVts-infected cells and visualized the dynamic localization of VSV-Gts and PtdIns4P in real time by time-lapse confocal video microscopy (Figure 4). VSVGts was labeled with a tagged Fab fragment of antiVSV-G antibody, and PtdIns4P formation was followed by using recombinant GFP-Fapp1-PH (GST-GFPFapp1-PH) added at noninhibitory concentrations (0.2 mM). At the beginning of the experiment, VSV-Gts resided in reticular ER membranes, and little apparent colocalization with the PtdIns4P reporter was observed. GFPFapp1-PH efficiently labeled the Golgi complex (Weixel et al., 2005), which remained intact throughout the experiments (Figure 4). ER export was initiated with the typical lag period of 5–7 min (Figures 4B and 4C) required for VSV-Gts trimerization (Doms et al., 1987). Export was detected by the elevation of VSV-Gts fluorescence intensity on ER membranes, reflecting the initial concentration of VSV-Gts at ERES (Figure 4, and Movie S1). Despite sequential pair-image acquisition, limited sampling and single plane analysis utilized to ensure the measurement of colocalization, PtdIns4P transiently colocalized with VSV-Gts at ERES. At early time points, colocalization of PtdIns4P and VSV-Gts was clearly observed at peripheral ERES that reside on distinct reticular membranes remote from the juxtanuclear Golgi complex (Figure 4C). At later time points, VSV-Gts was depleted from the ER and efficiently mobilized to punctate VTCs, which for the most part did not colocalize with Fapp1-PH (Figures 4D and 4E). Transient localization of PtdIns4P at reticular ER sites where VSV-Gts concentrates was also observed in subsequent rounds of VSV-Gts export from the ER (Movie S1). The ability to visualize transient association of VSV-Gts with PtdIns4Pcontaining ER membranes during the synchronized export of VSV-Gts from the ER provides direct evidence to suggest that dynamic and localized lipid remodeling at ERES supports vesicular traffic from the ER. Developmental Cell 676 Figure 4. Dynamics of PtdIns4P Formation at ER Export Sites Infected cells that accumulated VSV-Gts at 40 C in the ER were permeabilzed and incubated on ice for 20 min in the presence of an Alexa 594-labeled Fab fragment of a monoclonal antibody to VSV-Gts. The cells were washed and shifted to 32 C to initiate ER export in the presence of cytosol and GFP-Fapp1-PH-domain (0.2 mM). Fluorescence pair images were acquired every 2 min by time-lapse spinning disc confocal video microscopy. Sample times (min) are indicated for each panel on the right. Arrows indicate colocalization between VSV-Gts and PtdIns4P at ERES. The area labeled with the large arrowhead in (B), (C), and (D) is enlarged in the inserts. Note colocalization of VSV-Gts and PtdIns4P at sites of initial VSV-Gts concentration (B and C) during the mobilization of VSV-Gts from the reticular ER (A) to mature VTCs (E). Transient colocalization of PtdIns4P reporter with VSV-Gts is mainly observed in the initial stages of VSV-Gts trimerization and ER export (B and C). Scale bar is 5 mm. Coat Assembly Is Regulated by PtdIns4P VSV-Gts is mobilized through ERES by the activity of the COPII coat. We therefore analyzed the colocalization of Fapp1-PH with ERES stably coated with COPII. COPII assembly on ERES was stabilized by the addition of Sar1-GTP in semi-intact cells in reactions supplemented with cytosol and GFP-Fapp1-PH. The localization of PtdIns4P (using GFP-Fapp1-PH internal fluorescence) with respect to ERES (Sec23) and the Golgi complex (mannosidase II) was determined by confocal microscopy (Figure 5A). GFP-Fapp1-PH strongly labeled the juxtanuclear Golgi complex (Weixel et al., 2005). COPII coated ERES were localized within the Golgi region and throughout the cell periphery. Substantial colocalization between ERES and PtdIns4P was observed. To quantify the colocalization of PtdIns4P at ERES, permeabilized cells were incubated with GST-Fapp1-PH, fixed, and probed for Sec23 and GST-Fapp1-PH localization (Supplemental Experimental Procedures). 35% 6 1.5% (SEM, n = 55 cells) of peripheral Sec23-positive sites were colabeled with the Fapp1-PH domain. Thus, in agreement with the dynamic localization of VSV-Gts and Fapp1-PH on reticular ER during synchronized ER exit of VSV-Gts (Figure 4), PtdIns4P is localized to ERES. Can PtdIns4P be utilized to support COPII assembly? We analyzed the effect of PtdIns4P sequestration on Sar1-induced Sec23/24 recruitment to microsome membranes. Under conditions in which ER export is inhibited, GST-Fapp1-PH or neomycin did not affect Sar1induced Sec23 membrane binding (not shown). To focus on the role of membrane associated PI-kinases in regulating COPII assembly, we analyzed the recruitment of purified Sec23/24 subunits by Sar1-GTP, in reactions in which we restricted the levels of ATP (1 mM ATP instead of an ATP-regeneration system) to reduce the activity of lipid kinases (Figure 5B). These limiting conditions significantly reduce Sar1-GTP dependent COPII Regulation of ER Export by Phosphoinositides 677 assembly, but under these conditions, GST-Fapp1-PH inhibited Sec23 recruitment (K1/2 of 12–15 mM). Furthermore, inhibition of Sar1-dependent coat recruitment by GST-Fapp1-PH (K1/2 of 8 mM) was also observed in assays reconstituted with cytosol and restricted levels of ATP, in which the membranes were subjected to high salt wash (2.5 M urea) (Figure 5C). Therefore Sec23/24 recruitment to membranes is assisted by PtdIns4P. However, the sensitivity of Sar1-dependent Sec23/24 recruitment to PtdIns4P sequestration by Fapp1-PH is lower than that observed for ER export (Figure 1). Can PI 4-kinase activity explain the dependency of COPII assembly on ATP? We analyzed the ability of PtdIns4P to support ATP-independent recruitment of Sec23. Membranes were incubated with PtdIns4P micelles or with ATP or an ATP-regenerating system as controls. Sar1-dependent Sec23 recruitment required ATP and was markedly enhanced when an ATP-regenerating system was added (Figure 5D). Addition of PtdIns4P led to ATP-independent Sar1-induced recruitment of Sec23/24. The added PtdIns4 micelles associated with or delivered PtdIns4P to ER membranes. In either case, added PtdIns4P provided binding sites for the mammalian COPII, akin to the role of PtdIns4P in supporting the assembly of yeast COPII on synthetic liposomes (Matsuoka et al., 1998). PtdIns4P micelles did not support robust Sec23 recruitment. Therefore dynamic PtdIns de- and rephosphorylation reactions may regulate COPII assembly. Alternatively, additional ATPdependent steps that are independent of PtdIns phosphorylation may operate to facilitate COPII assembly. The nucleation of COPII at ERES, rather than COPIImembrane binding, may be affected by PtdIns4P sequestration. We therefore used IF to examine the effect of PtdIns4P sequestration on Sar1-induced nucleation of COPII at ERES. COPII assembly was reconstituted in semi-intact cells by the addition of cytosol and Sar1-GTP in the presence of ATP-regeneration system. Sar1-GTP induced robust recruitment of both Sec23 (COPII inner layer) and Sec13 (COPII outer layer) to defined punctate sites (Figures 5E and 5F). Fapp1-PH domain (Figure 5E) (5 mM) or neomycin (Figure 5F) (1 mM), which did not affect COPII recruitment to microsome membranes (see above), markedly reduced Sar1induced nucleation of both COPII layers at ERES. It could be that methodological differences between the two assays resulted in the observed enhanced sensitivity of COPII recruitment to PtdIns4P sequestration as detected by IF. Alternatively, the inhibition of COPII recruitment in the morphological assay may reflect inhibition of COPII nucleation at ERES, whereas the overall levels of recruited COPII as measured on microsome membranes were mildly affected. PtdIns4P Marks ERES and Is Required to Support Carrier-Membrane Fission Interfering with the dynamics of ERES assembly may result in the formation of stabilized ERES intermediates that precede coat assembly and accumulate PtdIns4P. We therefore generated enlarged ERES for further morphological analysis. Permeabilized VSV-Gts-expressing cells were incubated with Sar1-GTP in the absence of cytosol. Under these conditions, highly elongated and constricted Sar1-induced ERES that selectively concen- trate cargo are formed (see VSV-Gts, Figures 6B and 6D) (Aridor et al., 2001). These tubular transport intermediates are formed independently and prior to COPII coat assembly. Sar1 is targeted exclusively to these elongated tubules that are well segregated from the Golgi complex (Figure 6A). Sar1-GTP-dependent tubules were generated in the presence of low concentrations of either GST-Fapp1-PH or anti-PtdIns4P antibodies, which provide an alternative independent probe for PtdIns4P localization. PtdIns4P visualized by both PtdIns4P reporters decorated VSV-Gts containing elongated tubular ERES (Figures 6B and 6D) as well as Golgi structures (arrowheads in Figure 6B and not shown). The distinct colocalization between PtdIns4P and cargo in tubular export domains suggests that the lipid composition of ERES differ from that of the bulk of ER membranes. To analyze the possible contribution of PtdIns4P to the formation of Sar1-dependent tubular ERES, we increased the concentration of the GSTFapp1-PH domain from 0.6 mM to 5 mM (Figure 6C). Strikingly, while export sites marked by the enrichment of VSV-Gts (Figure 6C) and Sar1 (not shown) were still observed, the elongation of these domains into tubular structures was prevented. The colocalization between VSV-Gts and the PtdIns4P reporter was preserved. Similar results were obtained with neomycin (see Sar1-decorated tubules, Figure 6E). We have recently provided evidence to suggest that Sar1-mediated membrane constriction and tubulation at ERES controls vesicle fission (Bielli et al., 2005). Thus, PtdIns4P in ERES tubules may support Sar1-induced tubule constriction and elongation, a step that precedes vesicle fission. Discussion The concentration and export of cargo via organized ERES provides a facet of selectivity to the ER-sorting machinery of higher eukaryotic cells (Aridor et al., 2004; Bevis et al., 2002). The molecular basis for ERES formation and function is unknown. We hypothesized that domains of distinct lipid composition are established in fluid ER membranes to define ERES. We now demonstrate that PtdIns4P-containing domains are dynamically generated to regulate ERES membrane deformation and coat nucleation to support ER export. Several independent lines of evidence support our conclusions. A specific PtdIns-binding domain that sequesters PtdIns4P inhibited ER export (Figure 1). Structure-function analysis and lipid-binding competition correlated the inhibitory effects of the domain with specific lipid recognition (Figure 2 and Figure S1). The cytosolic domain of the ER-localized PtdIns4P phosphatase Sac1 delayed ER export (Figure 2). Direct analysis of PtdIns phosphorylation on ER membranes demonstrated that recruitment and activation of Sar1, which initiates ER export, transiently elevated PtdInsP and PtdInsP2 levels (Figure 3). These biochemical results correlated well with in vitro real-time imaging, which demonstrated that PtdIns4P accumulated transiently and locally during the concentration of cargo at ERES at sites of COPII assembly and membrane constriction (Figures 4, 5, and 6). The recognition that PtdIns are regulators of endocytic traffic led to the identification of PtdIns(4,5)P2-binding Developmental Cell 678 Figure 5. The Recruitment of Sec23 to ERES Is Assisted by PtdIns4P (A) Permeabilized cells were incubated in the presence of Sar1-GTP (6 mg, 220 ml final volume), RLC, and GFP-Fapp1-PH (3 mM) for 30 min at 32 C. The cells were fixed and stained for Sec23 and mannosidase II (Man II) as indicated. The localization of GFP-Fapp1-PH (by GFP fluorescence) ERES (Sec23, red) and the Golgi complex (Man II, blue) was determined. Merge images for Man II and GFP-Fapp1-PH, Sec23 and Man II, and a merged image for all three markers are shown. Arrows indicate colocalization of Fapp1-PH with peripheral Sec23-containing ERES. Scale bar is 5 mm. (B) Microsomes were incubated with purified Sec23/24 complex (0.7 mg, 60 ml final volume) and 1 mM ATP at 32 C for 15 min in the presence or absence of Sar1-GTP (0.25 mg) and GST-Fapp1-PH domain as indicated. The membrane recruitment of Sec23 was determined by western blot with affinity-purified anti-Sec23 antibody. (C) Microsomes were washed in buffer containing 2.5 M urea for 30 min on ice. The membranes were reisolated by centrifugation and incubated with RLC and 1 mM ATP as in (B), in the presence or absence of Sar1-GTP (0.1 mg) and GST-Fapp1-PH as indicated, and membrane recruitment of Sec23 was determined. (D) Microsomes were incubated with RLC at 32 C for 15 min in the presence or absence of Sar1-GTP (0.3 mg, 60 ml final volume), ATP-regeneration system (indicated as reg.), 1 mM ATP, or 50 mM PtdIns4P micelles as indicated, and the recruitment of Sec23 was determined. Data are representative of three independent experiments. Regulation of ER Export by Phosphoinositides 679 Figure 6. PtsIns4P Is Formed and Accumulated on Sar1-Induced Tubules (A) Permeabilized cells (220 ml final volume) were incubated in the presence of Sar1-GTP (6 mg) for 30 min at 32 C. The cells were fixed and stained for Sar1 and giantin (merged images are shown as indicated). (B and C) VSVts-infected cells were permeabilized cells and incubated as in (A) in the presence of 0.6 mM (B) or 5 mM (C) of GST-Fapp1-PH for 30 min at 32 C to form VSV-Gts-containing tubular ERES. The cells were fixed and stained with VSV-Gts and GST-Fapp1-PH as indicated. (D) Permeabilized cells were incubated with anti-PtdIns4P antibodies as in (A). The cells were fixed and further stained for VSV-Gts. The localization of VSV-Gts and the anti-PtdIns4P antibody was determined by IF. A projection of five consecutive optical sections is presented. Bars are 10 mm. Note the colocalization of VSV-Gts and PtdIns4P reporters on Sar1 tubular domains (arrow in [B]). Arrowheads in (B) point to juxtanuclear Golgi elements positive for Fapp1-PH. (E) Permeabilized cells were incubated as in (A) in the presence or absence of neomycin (1 mM). The cells were fixed stained for Sar1. Note inhibition of Sar1 tubule elongation with neomycin or Fapp1-PH. accessory proteins that support clathrin-mediated endocytosis (Wenk and De Camilli, 2004). PtdIns(4,5)P2 may function in place of small GTPases to support the membrane binding of AP-2/clathrin. However, PtdIns4P is required for ARF1 regulated AP-1/clathrin membrane binding and both PtdIns4P and PtdIns(4,5)P2 are required to support VSV-G exit from the TGN (Wang et al., 2003). Our studies suggest that utilization of PtdIns signals may be a general mechanism to define sites for regulated cargo sorting and vesicle formation. Both PtdInsP and PtdInsP2 levels were elevated by Sar1 activation on ER membranes (Figure 3). Membrane-associated type II PI 4-kinase is activated by Sar1 in the absence of cytosol under conditions that do not support efficient formation of PtdInsP2. Accord- ingly, the PtdIns(4,5)P2-binding domain of PLCd1-PH effectively inhibited ER export only in the presence of cytosol under conditions that promote efficient PtdInsP2 formation (Figure 1 and 3). Thus, PtdIns4P itself is a signal that regulates ER export. However, subsequent formation of PtdIns (4,5)P2 may be utilized in a positive feedback loop activating PLD (Pathre et al., 2003) and enhancing COPII vesicle formation. Active populations of type II PI 4-kinase a and b are localized to the ER (Waugh et al., 2003; Wei et al., 2002). Future studies will address the possible role of these enzymes in regulating ER export. Interestingly, type II PI 4-kinases require detergent-induced perturbation of membranes for activation. Sar1 utilizes its NH2 terminal amphipathic domain to perturb and deform membranes (E–F) Permeabilized cells (220 ml final volume) were incubated with RLC and ATP-regeneration system in the presence or absence of Sar1-GTP (6 mg) and GST-Fapp1-PH domain (5 mM) (E) or neomycin (1 mM) (F) as indicated for 30 min at 32 C. The cells were fixed and stained for Sec23 (E) or Sec23 and Sec13 (F). Images for each experiment were acquired under identical conditions. Scale bar is 5 mm. Developmental Cell 680 (Bielli et al., 2005; Lee et al., 2005). An intriguing possibility is that the membrane-deforming activity of Sar1 provides a mechanism to couple Sar1-induced membrane curvature with the activation of PI 4-kinase signaling required to regulate ER export. While the major PI 4-kinase activities in budding yeast (PiK1 and Stt4) are not localized to the ER and do not regulate ER export (Audhya et al., 2000), recent studies have demonstrated that deletion of the phosphatase Sac1, which is mainly localized in the ER, leads to elevated levels of PtdIns4P in yeast ER (Tahirovic et al., 2005). Elevation of PtdIns4P due to rapid inactivation of Sac1p does not inhibit ER export, yet prolonged imbalance of PtdIns4P hydrolysis in Sac1-deleted strains culminate in defects in ER-to-Golgi transport (Foti et al., 2001). Sac1 mutations display negative genetic interactions with genes encoding for COPII inner and outer layers (Sec23 and Sec13) (Cleves et al., 1989). Therefore, PtdIns4P may regulate some aspects of the yeast ER. However, the early secretory pathway in budding yeast markedly differs from that of mammalian cells as it lacks organized ERES and Golgi structures. Organized mammalian ERES may have evolved to further utilize PtdIns signals. The transient nature of PtdIns4P formation observed in our morphological and biochemical analysis suggests that tight spatial and temporal control of PtdIns4P levels by both PI kinases and phosphatases is required to regulate the nucleation of COPII at ERES on the fluid ER membrane. Lipid signals have previously been implicated in ERES function (Fabbri et al., 1994; Lee et al., 2004; Nagaya et al., 2002; Pathre et al., 2003; Runz et al., 2006; Shimoi et al., 2005). In addition to PtdIns (current work), PA, diacylglycerol (DAG), and sterols regulate ER export. DAG kinase d may regulate ER export, and PLCd4 that generates DAG is localized to the ER. p125, a PA-preferring phospholipase A1 homolog that binds Sec23, is recruited by Sar1 to regulate the cellular localization of ERES. Therefore, PtdIns signals can be viewed as part of an extensive lipid remodeling cascade that regulates ER export. How is the PtdIns4P signal decoded at ERES? COPII assembly is assisted by PA, and a preference for PtdIns4P was revealed for Sar1-dependent COPII binding to synthetic liposomes (Matsuoka et al., 1998; Pathre et al., 2003). Thus, COPII subunits may participate in decoding the PtdIns4P signal. However, our analysis suggests that the information encoded by PtdIns4P is also utilized in steps that precede coat binding perhaps by accessory proteins that assist in ERES assembly. Our results suggest that the lipid composition of ERES may resemble that of the Golgi complex rather than that of the bulk ER and thus may utilize functionally similar PtdIns regulated proteins for organization. One possible accessory protein that regulates the Golgi structure in a PtdIns-dependent manner is b III spectrin (Siddhanta et al., 2003). Fragments of spectrin inhibit ER export in vivo and in vitro (Devarajan et al., 1997; Godi et al., 1998). Lipid transfer proteins play a key role in regulating lipid distribution at the Golgi complex. Some, including FFAT-motif-tagged proteins like the PH-domain containing OSBP-related protein 9, localize to ERES (Wyles and Ridgway, 2004), and their ER localized receptors VAP-B and VAP-A participate in regulating the organiza- tion of the ER and ER export (Amarilio et al., 2005). Therefore, a possible role for such receptors and members of their PtdIns-interacting protein ligands in regulating the micro-organization of ER membranes and ER export may be envisioned. Our results point to a functional role for PtdIns4P in the formation and elongation of Sar1-induced tubules that proliferate from ERES and serve as intermediates in vesicle fission (Aridor et al., 2001; Bielli et al., 2005). Fission-intermediate tubular domains have also been observed during export from the TGN (Liljedahl et al., 2001). Fapp proteins (Fapp1 and Fapp2) localize to the TGN through PtdIns4P binding and propagate the formation of TGN tubules to control TGN export (Godi et al., 2004). PtdIns4P may regulate functionally homologous PtdIns4P-binding Fapp-like proteins to control tubule elongation at ERES. Such proteins may utilize lipid binding/transfer activities to achieve local lipid composition favorable for coat nucleation and vesicle fission (Vieira et al., 2005). The targets that decode PtdIns4P at ERES remain to be defined. The demonstration of localized formation and function of PtdIns4P at ERES provides a basis to study how these unique domains are established within the ER membrane to support and regulate ER export. Experimental Procedures Materials Recombinant proteins and antibodies utilized in the study are described in Supplemental Data. Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), and brain PtdIns were from Sigma. Dilauryl-PA, PtdIns, PtsIns4P, and PtdIns(4,5)P2 were from Avanti polar lipids. Lipid arrays and PtdIns4P utilized for micelle preparations were from Echelon, g-32P-ATP (3000 Ci/mmol) from ICN, TLC plates were from Voigt Global Distribution, LLC, Digitonin from Wako, and Malachite Green Phosphate assay kit from BioAssay Systems. Tissue culture reagents were from Gibco. Other reagents were from Sigma. Infection of NRK Cells NRK cells were infected with tsO45 VSV as described (Plutner et al., 1992). Preparation of Membrane Microsomes Microsomes from VSVts-infected or -uninfected NRK cells were prepared as described (Rowe et al., 1996). Coat Recruitment Assay Sec23 recruitment assays were performed as described (Aridor and Balch, 2000; Aridor et al., 1998). Salt wash details are described in the Supplemental Data. ER-Vesicle Budding Assays COPII budding assays were performed as described (Aridor et al., 1998; Rowe et al., 1996). Semi-Intact Cell Morphological Analysis and Microscopy Morphological analysis of ER export was performed as described (Aridor et al., 2001). Images were acquired on an Olympus Fluoview 500 confocal microscope. Real-time imaging conditions are described in the Supplemental Data. Quantitation of colocalization between GST-Fapp-PH with Sec23 and the mobilization of VSV-Gts to VTCs is described in the Supplemental Data. Alexa 594-tagged Fab fragment of anti-VSV-G antibody (P5D4) was added during incubations to label VSV-G in ER export assays, which quantify the effects of Sac1 on mobilization of VSV-Gts to VTCs. Regulation of ER Export by Phosphoinositides 681 Measurement of Phosphoinositide Formation on Isolated ER Membranes Measurement of phosphoinositide formation on isolated ER membranes is described in the Supplemental Data. Measurement of Phosphatase Activity of Soluble Sac1 Phosphatase activity of Sac1 was determined as described (Maehama et al., 2000). Supplemental Data The Supplemental Data include analysis of lipid-binding properties of wt and mutated Fapp1-PH domain (Figure S1), properties of fractionated rat liver membranes (Figure S2), and the dynamics of PtdIns4P formation at ER exit sites depicted in a movie. The supplemental text includes additional details on experimental procedures utilized in the study. Supplemental Data are available at http:// www.developmentalcell.com/cgi/content/full/11/5/671/DC1/. Acknowledgments We thank Drs. T. Balla (National Institutes of Health), W.E. Balch (The Scripps Research Institute), M.A. Lemmon (University of Pennsylvania), and G.S. Taylor (University of Nebraska) for valuable reagents. The study was supported by grants from the Edward Mallinckrodt Jr. Foundation (M.A.), NIH DK062318 (M.A.), NIH DK064613 (O.A.W.), American Heart Association (A.B.P.), and NIH T32-DK61296 (K.M.W.). Received: June 28, 2005 Revised: June 25, 2006 Accepted: September 4, 2006 Published: November 6, 2006 References Amarilio, R., Ramachandran, S., Sabanay, H., and Lev, S. (2005). Differential regulation of ER structure through VAP-Nir protein interaction. J. Biol. Chem. 280, 5934–5944. Antonny, B., and Schekman, R. (2001). ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol. 13, 438–443. Aridor, M., and Balch, W.E. (2000). Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J. Biol. Chem. 275, 35673–35676. Aridor, M., Bannykh, S.I., Rowe, T., and Balch, W.E. (1995). Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to golgi transport. J. Cell Biol. 131, 875–893. Aridor, M., Weissman, J., Bannykh, S., Nuoffer, C., and Balch, W.E. (1998). Cargo selection by the COPII budding machinery during export from the ER. J. Cell Biol. 141, 61–70. Aridor, M., Fish, K.N., Bannykh, S., Weissman, J., Roberts, T.H., Lippincott-Schwartz, J., and Balch, W.E. (2001). The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol. 152, 213–229. Bevis, B.J., Hammond, A.T., Reinke, C.A., and Glick, B.S. (2002). De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nat. Cell Biol. 4, 750–756. Bielli, A., Haney, C.J., Gabreski, G., Watkins, S.C., Bannykh, S.I., and Aridor, M. (2005). Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J. Cell Biol. 171, 919–924. Cleves, A.E., Novick, P.J., and Bankaitis, V.A. (1989). Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J. Cell Biol. 109, 2939–2950. De Matteis, M.A., and Godi, A. (2004). PI-loting membrane traffic. Nat. Cell Biol. 6, 487–492. Devarajan, P., Stabach, P.R., De Matteis, M.A., and Morrow, J.S. (1997). Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc. Natl. Acad. Sci. USA 94, 10711–10716. Doms, R.W., Keller, D.S., Helenius, A., and Balch, W.E. (1987). Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J. Cell Biol. 105, 1957– 1969. Dowler, S., Currie, R.A., Campbell, D.G., Deak, M., Kular, G., Downes, C.P., and Alessi, D.R. (2000). Identification of pleckstrinhomology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351, 19–31. Fabbri, M., Bannykh, S., and Balch, W.E. (1994). Export of protein from the endoplasmic reticulum is regulated by a diacylglycerol/ phorbol ester binding protein. J. Biol. Chem. 269, 26848–26857. Foti, M., Audhya, A., and Emr, S.D. (2001). Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol. Biol. Cell 12, 2396–2411. Godi, A., Santone, I., Pertile, P., Devarajan, P., Stabach, P.R., Morrow, J.S., Di Tullio, G., Polishchuk, R., Petrucci, T.C., Luini, A., and De Matteis, M.A. (1998). ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc. Natl. Acad. Sci. USA 95, 8607– 8612. Godi, A., Pertile, P., Meyers, R., Marra, P., Di Tullio, G., Iurisci, C., Luini, A., Corda, D., and De Matteis, M.A. (1999). ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1, 280–287. Godi, A., Di Campli, A., Konstantakopoulos, A., Di Tullio, G., Alessi, D.R., Kular, G.S., Daniele, T., Marra, P., Lucocq, J.M., and De Matteis, M.A. (2004). FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 6, 393–404. Hughes, W.E., Woscholski, R., Cooke, F.T., Patrick, R.S., Dove, S.K., McDonald, N.Q., and Parker, P.J. (2000). SAC1 encodes a regulated lipid phosphoinositide phosphatase, defects in which can be suppressed by the homologous Inp52p and Inp53p phosphatases. J. Biol. Chem. 275, 801–808. Kuehn, M.J., Herrmann, J.M., and Schekman, R. (1998). COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature 391, 187–190. Aridor, M., Guzik, A.K., Bielli, A., and Fish, K.N. (2004). Endoplasmic reticulum export site formation and function in dendrites. J. Neurosci. 24, 3770–3776. Lee, A., and Lemmon, M.A. (2001). Analysis of phosphoinositide binding by pleckstrin homology domain from dynamin. Methods Enzymol. 329, 457–468. Audhya, A., Foti, M., and Emr, S.D. (2000). Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11, 2673–2689. Lee, S.B., Varnai, P., Balla, A., Jalink, K., Rhee, S.G., and Balla, T. (2004). The pleckstrin homology domain of phosphoinositidespecific phospholipase Cdelta4 is not a critical determinant of the membrane localization of the enzyme. J. Biol. Chem. 279, 24362– 24371. Balla, A., Tuymetova, G., Barshishat, M., Geiszt, M., and Balla, T. (2002). Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 277, 20041–20050. Bannykh, S.I., Rowe, T., and Balch, W.E. (1996). The organization of endoplasmic reticulum export complexes. J. Cell Biol. 135, 19–35. Lee, M.C., Orci, L., Hamamoto, S., Futai, E., Ravazzola, M., and Schekman, R. (2005). Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122, 605– 617. Bannykh, S.I., Nishimura, N., and Balch, W.E. (1998). Getting into the Golgi. Trends Cell Biol. 8, 21–25. Lemmon, M.A., and Ferguson, K.M. (2001). Molecular determinants in pleckstrin homology domains that allow specific recognition of phosphoinositides. Biochem. Soc. Trans. 29, 377–384. Barlowe, C. (2003). Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol. 13, 295–300. Liljedahl, M., Maeda, Y., Colanzi, A., Ayala, I., Van Lint, J., and Malhotra, V. (2001). Protein kinase D regulates the fission of cell surface Developmental Cell 682 Maehama, T., Taylor, G.S., Slama, J.T., and Dixon, J.E. (2000). A sensitive assay for phosphoinositide phosphatases. Anal. Biochem. 279, 248–250. Wei, Y.J., Sun, H.Q., Yamamoto, M., Wlodarski, P., Kunii, K., Martinez, M., Barylko, B., Albanesi, J.P., and Yin, H.L. (2002). Type II phosphatidylinositol 4-kinase beta is a cytosolic and peripheral membrane protein that is recruited to the plasma membrane and activated by Rac-GTP. J. Biol. Chem. 277, 46586–46593. Matsuoka, K., Orci, L., Amherdt, M., Bednarek, S.Y., Hamamoto, S., Schekman, R., and Yeung, T. (1998). COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93, 263–275. Weixel, K.M., Blumental-Perry, A., Watkins, S.C., Aridor, M., and Weisz, O.A. (2005). Distinct golgi populations of phophatidylinositol 4-phosphate regulated by phosphatidylinositol 4-kinases. J. Biol. Chem. 280, 10501–10508. destined transport carriers from the trans-Golgi network. Cell 104, 409–420. Miller, E.A., Beilharz, T.H., Malkus, P.N., Lee, M.C., Hamamoto, S., Orci, L., and Schekman, R. (2003). Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114, 497–509. Mironov, A.A., Mironov, A.A., Jr., Beznoussenko, G.V., Trucco, A., Lupetti, P., Smith, J.D., Geerts, W.J., Koster, A.J., Burger, K.N., Martone, M.E., et al. (2003). ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev. Cell 5, 583–594. Mossessova, E., Bickford, L.C., and Goldberg, J. (2003). SNARE selectivity of the COPII coat. Cell 114, 483–495. Nagaya, H., Wada, I., Jia, Y.J., and Kanoh, H. (2002). Diacylglycerol kinase delta suppresses ER-to-Golgi traffic via its SAM and PH domains. Mol. Biol. Cell 13, 302–316. Palade, G. (1975). Intracellular aspects of the process of protein synthesis. Science 189, 347–358. Pathre, P., Shome, K., Blumental-Perry, A., Bielli, A., Haney, C.J., Alber, S., Watkins, S.C., Romero, G., and Aridor, M. (2003). Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export. EMBO J. 22, 4059–4069. Plutner, H., Davidson, H.W., Saraste, J., and Balch, W.E. (1992). Morphological analysis of protein transport from the ER to Golgi membranes in digitonin-permeabilized cells: role of the P58 containing compartment. J. Cell Biol. 119, 1097–1116. Rowe, T., Aridor, M., McCaffery, J.M., Plutner, H., Nuoffer, C., and Balch, W.E. (1996). COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J. Cell Biol. 135, 895– 911. Runz, H., Miura, K., Weiss, M., and Pepperkok, R. (2006). Sterols regulate ER-export dynamics of secretory cargo protein ts-O45-G. EMBO J. 25, 2953–2965. Shimoi, W., Ezawa, I., Nakamoto, K., Uesaki, S., Gabreski, G., Aridor, M., Yamamoto, A., Nagahama, M., Tagaya, M., and Tani, K. (2005). p125 is localized in endoplasmic reticulum exit sites and involved in their organization. J. Biol. Chem. 280, 10141–10148. Siddhanta, A., Radulescu, A., Stankewich, M.C., Morrow, J.S., and Shields, D. (2003). Fragmentation of the Golgi apparatus. A role for beta III spectrin and synthesis of phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 278, 1957–1965. Simonsen, A., Wurmser, A.E., Emr, S.D., and Stenmark, H. (2001). The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13, 485–492. Stephens, D.J. (2003). De novo formation, fusion and fission of mammalian COPII-coated endoplasmic reticulum exit sites. EMBO Rep. 4, 210–217. Tahirovic, S., Schorr, M., and Mayinger, P. (2005). Regulation of intracellular phosphatidylinositol-4-phosphate by the Sac1 lipid phosphatase. Traffic 6, 116–130. Vieira, O.V., Verkade, P., Manninen, A., and Simons, K. (2005). FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J. Cell Biol. 170, 521–526. Wang, Y.J., Wang, J., Sun, H.Q., Martinez, M., Sun, Y.X., Macia, E., Kirchhausen, T., Albanesi, J.P., Roth, M.G., and Yin, H.L. (2003). Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299–310. Waugh, M.G., Minogue, S., Anderson, J.S., Balinger, A., Blumenkrantz, D., Calnan, D.P., Cramer, R., and Hsuan, J.J. (2003). Localization of a highly active pool of type II phosphatidylinositol 4-kinase in a p97/valosin-containing-protein-rich fraction of the endoplasmic reticulum. Biochem. J. 373, 57–63. Wenk, M.R., and De Camilli, P. (2004). Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. USA 101, 8262–8269. Wyles, J.P., and Ridgway, N.D. (2004). VAMP-associated protein-A regulates partitioning of oxysterol-binding protein-related protein-9 between the endoplasmic reticulum and Golgi apparatus. Exp. Cell Res. 297, 533–547.