* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Neonatal Cardiology
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Echocardiography wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Williams syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Down syndrome wikipedia , lookup
Marfan syndrome wikipedia , lookup
DiGeorge syndrome wikipedia , lookup
Aortic stenosis wikipedia , lookup
Turner syndrome wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Congenital heart defect wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
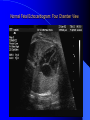
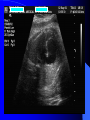
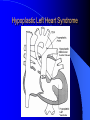
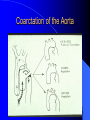
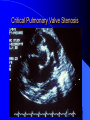
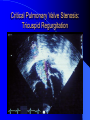
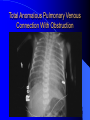
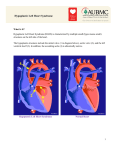
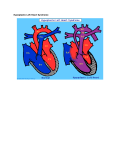
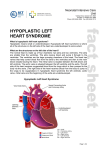
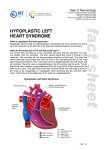
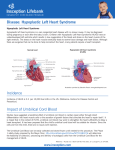
Diagnosis and Management of the Neonate With Critical Congenital Heart Disease Department of Pediatrics National Naval Medical Center 15 April 03 Neonate With Critical CHD Prenatal evaluation Initial neonatal evaluation and management Stabilization and transport Confirmation of the diagnosis Preoperative evaluation of non-cardiac organ systems Timing and type of surgery Lesion specific management Prenatal Assessment Obstetric history Genetic evaluations Prenatal ultrasound Fetal echocardiography – 60% of cardiology admissions at CHOP prenatally diagnosed – 49% of HLHS admissions at Children’s Hospital of Wisconsin were prenatally diagnosed Normal Fetal Echocardiogram: Four Chamber View Ebstein’s Anomaly Critical CHD: Initial Evaluation and Management ABC’s – Oxygen (judicial) to saturations of 80-85% – Place umbilical lines – PGE (0.025-0.1 micrograms/Kg/min) History Complete physical with four extremity BP’s Pre and post-ductal oxygen saturations Hyperoxia test CXR EKG Echocardiogram Suspected CHD: Initial Evaluation and Management Pre and post-ductal oxygen saturations – If pre-ductal sat higher than post-ductal sat (differential cyanosis) Left heart abnormalities (such as aortic arch hypoplasia, critical aortic stenosis, interrupted aortic arch) Persistent pulmonary hypertension – If post-ductal sat higher than pre-ductal (reverse differential cyanosis) TGA with CoA or TGA with IAA TGA with supersystemic pulmonary vascular resistance Stabilization and Transport ABC’s Place lines (UVC, UAC) Check and administer glucose and calcium as needed If severe respiratory distress, shock, or severe cyanosis: sedate, paralyze, intubate, and mechanically ventilate to oxygen sats of 80-85%. Place NG tube. Check ABG’s Sepsis evaluation. Antibiotics Stabilization and Transport PGE1 lowest dose possible (0.025 micrograms/kg/min) Judicious use of pressors – Dopamine and Dobutamine – Milrinone Consider use of 2-3% CO2 in ventilated patients with left sided obstructive lesions Side effects of PGE1 More common in premature infants Clinical deterioration if pulmonary venous obstruction present – HLHS with restrictive/intact atrial septum – TGA with intact ventricular septum and a restrictive/intact atrial septum – TAPVR with obstruction Apnea Hypotension Confirmation of the Diagnosis Echocardiography – primary diagnostic modality for anatomic definition – Not “non-invasive” in sick newborn Cardiac catheterization – Rarely indicated to confirm diagnosis – Therapeutic (interventional procedures) Genetic Evaluation Genetic – Trisomies 13, 18, 21 – Monosomy X (Turner’s syndrome): Coarctation – 22q11 Deletion (DiGeorge syndrome): Conotruncal abnormalities – 7q11 Deletion (Williams syndrome) – Single gene defects (Noonan’s, Holt-Oram, Ellis-van Crevald, Alagille) Unknown cause – Vacterl – Charge Evaluation of Other Organ Systems CNS: CNS anomalies and ischemic injury GI: risk for NEC Renal: 3-6% incidence of urinary tract anomalies Timing and Type of Surgery Cardiac catheterization procedures – Balloon atrial septostomy – Balloon valvuloplasty – Balloon angioplasty Open versus Closed Palliative versus Corrective – Trend towards early, corrective surgery, even in preterm or low birth weight infants Critical CHD: Lesion Specific Management Ductal dependent for systemic blood flow – HLHS management Ductal dependent for pulmonary blood flow D-transposition of the great arteries Total anomalous pulmonary venous connection with obstruction Hypoplastic Left Heart Syndrome Hypoplastic Left Heart Syndrome: Pathology: aortic atresia/severe stenosis, mitral atresia/severe stenosis, hypoplastic left ventricle and aortic arch. 1.5% of congenital heart defects. Most common cause of cardiac related neonatal mortality. Ductal dependent for systemic blood flow at birth Patients may have associated chromosomal or developmental abnormalities Hypoplastic Left Heart Syndrome: Clinical Presentation May be diagnosed by fetal ultrasound. Prognostic issues: atrial septal position, size of foramen ovale (if restrictive, pulmonary venous obstruction) Classic presentation: cardiogenic shock, poor perfusion, decreased pulses, profound metabolic acidosis. May have systolic murmur. Diagnosis: echocardiogram. CXR and EKG are non-specific. Hypoplastic Left Heart Syndrome: Initial Medical Management Prostaglandin E1 0.025 to 0.2 micrograms/kg/min- watch for side effects Room air ventilation: ideal ABG ph 7.4/ pco2 40/ po2 40 Inhaled CO2 to manipulate pulmonary vascular resistance? Watch the use of pressors- may be harmful Hypoplastic Left Heart Syndrome: Stage One Norwood Performed in neonatal period Procedure: MPA divided; distally MPA closed with patch; hypoplastic aortic arch reconstructed and anastomosed to the proximal MPA with homograft augmentation; atrial septosomy; systemic shunt placed. Hypoplastic Left Heart Syndrome: S/P Stage One Norwood Surgical issues: – Unobstructed aortic arch – Adequate atrial septectomy – Balanced pulmonary and systemic blood flow (Qp:Qs 1:1) Survival at major centers: 80% Hypoplastic Left Heart Syndrome: HemiFontan Procedure Shunt ligated, superior vena cava anastomosis to pulmonary artery, pulmonary arteries augmented, flap of tissue closes SVC-RA junction Performed around 6 months of age following Norwood Volume load on right ventricle removed Excellent survival statistics Hypoplastic Left Heart Syndrome: Fontan Procedure Performed around 1824 months Venous and systemic circulations are separated Survival: excellent Long term issues: RV function, arrhythmias Hypoplastic Left Heart Syndrome: Fenestrated Fontan Procedure Transplant in Hypoplastic Left Heart Syndrome Issues of waiting for donor heart Excellent operative results Limited donor availability Issues of life long immunosuppresion Coarctation of the Aorta Critical Pulmonary Valve Stenosis Critical Pulmonary Valve Stenosis: Tricuspid Regurgitation Ebstein Anomaly D-transposition of the Great Arteries Arterial Switch Procedure for D-TGA Total Anomalous Pulmonary Venous Connection Total Anomalous Pulmonary Venous Connection With Obstruction Total Anomalous Pulmonary Venous Connection With Obstruction