* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Aortic atresia
History of invasive and interventional cardiology wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Artificial heart valve wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Marfan syndrome wikipedia , lookup
Turner syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Coronary artery disease wikipedia , lookup
Aortic stenosis wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Myocardial infarction wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial septal defect wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
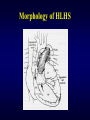
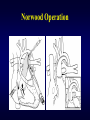
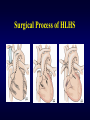
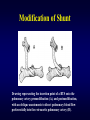
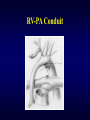
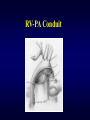
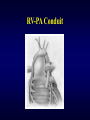
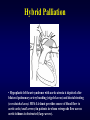
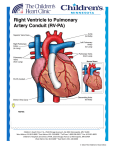
Aortic Atresia or Hypoplastic Left Heart Physiology Seoul National University Hospital Department of Thoracic & Cardiovascular Surgery Hypoplastic Left Heart Syndrome • Definition A developmental anomaly in which the aortic valve is atretic and the ascending aorta is hypoplastic * With few exceptions, there is in additional mitral atresia or mitral valve hypoplasia, severe left ventricular hypoplasia, & intact ventricular septum, called Hypoplastic left heart syndrome. • History Canton : 1st description of aortic atresia in 1850 Brockman : Coexisting maldevelopment of left side of heart Noonan & Nadas : Hypoplastic left heart syndrome in 1958 Cayler ; RPA-ascending aorta, bilateral PA bands in 1977 Norwood : Neonatal reconstructive surgery of HLHS in 1980 Bailey : Allograft heart transplantation in 1985 Hypoplastic Left Heart Physiology 1. Inability of the left heart to sustain an adequate cardiac output following birth because of underdevelopment of one or more left heart structures despite surgical or medical intervention 2. The term hypoplastic left heart physiology rather than the more common and entrenched term hypoplastic left heart syndrome because it more accurately describes the entities 3. Definition of hypoplastic left heart is physiologic, not morphologic, despite morphologic abnormalities being the underlying cause of left heart inadequancy. 4. An important implication of the term hypoplastic left heart physiology is that the left heart is incapable of sustaining systemic cardiac output, thereby limiting therapeutic options to reconstructions of single ventricular pumping or to transplantation Hypoplastic Left Heart Syndrome Classification Class I : Isolated cardiac anomaly Class II : Two congenital anomalies affecting left ventricular outflow Class III : More than two anomalies, or two with coexisting LV or ascending aortic or aortic arch hypoplasia Class IV : Aortic atresia * Anomalies : Congenital MV disease LV hypoplasia. Subvalvar, valvar, supravalvar AS Ascending aortic or arch hypoplasia IAA or COA Hypoplastic Left Heart Syndrome Pathophysiology • HLHS is characterized by marked hypoplasia or absence of the left ventricle, and severe aortic hypoplasia places the work of the pulmonary and systemic perfusion solely on the right ventricle. • Pulmonary venous return enters the right atrium via an interatrial communication and systemic perfusion is dependant on a PDA. • Cyanosis, congestive heart failure, and systemic hypoperfusion results as pulmonary vascular resistance decreases and pulmonary blood flow increases at the expense of systemic blood flow. Hypoplastic Left Heart Syndrome Morphogenesis & morphology • Currently, it is not clear whether the etiology of hypoplastic left heart physiology is similar an all cases. • Primary morphologic abnormalities at the aortic valve, mitral valve level, left ventricular myocardial level, or atrial septal level could all in theory lead ultimately to hypoplasia of the entire left side of the heart as gestation progresses • In hypoplastic left heart physiology, the heart is enlarged to about twice normal weight for age. • Among the 4 major categories defined by the left-sided valves, aortic atresia and mitral atresia is the most common form , representing two third, & aortic stenosis with mitral atresia is the least common form ( 5% ). Morphology of HLHS Morphology of HLHS Hypoplastic Left Heart Syndrome Morphology 1. Aortic valve & ascending aorta Absent valve, diminutive sinus, coarctation in 80% 2. Left ventricle & mitral valve Hypoplastic & intact ventricular septum in 95% Mitral valve atretic in 1/3, hypoplastic in 2/3 Variable degree of endocardial fibroelastosis, hypertropic muscle Normal LV cavity with VSD in 5% 3. Right ventricle ; enlarged and diffuse hypertrophy, variable TR 4. Atria & atrial septum Left atrium : small, thick walled, Right atrium : large Atrial septum : thick, interatrial communication ( PFO) 5. Other associated cardiac anomalies ; Uncommon Juxtaposed atrial appendages & double tricuspid orifice 6. Associated noncardiac anomalies ; frequently ( 25 -40%) Hypoplastic Left Heart Syndrome Clinical Features & Diagnosis 1. 2. 3. 4. 5. 6. 7. Mild cyanosis & respiratory distress in newborn Rapid deterioration with CHF & death within a week of birth, ductal closure is inevitable with variable times Hyperactive RV precordial impulse & midsystolic murmur with S2 accentuation, poor peripheral pulse Chest X ray : cardiomegaly with pulmonary plethora EKG : RVH, RAD Echocardiography Cardiac catheterization & cineangiography Hypoplastic Left Heart Syndrome Natural history 1. Incidence 4th common CHD ( 4~7% of CHD), 70% male 2. Death 40% survive beyond neonatal period, Uncommon survival beyond 6 weeks of age 25% of cardiac deaths during 1st week of life 15% of cardiac death during 1st month of life 3. Perinatal survival is dependent on PDA 4. Associated genetic & extracardiac anomalies in 20~40% (Chromosomal & major, minor CNS abnormalities, esp. micrencephaly in 25% in autopsy) Hypoplastic Left Heart Syndrome Preoperative management • After diagnosis, resuscitation & PGE1 is initiated • Avoiding supplemental inspired oxygen is critical to overall strategy; 21% oxygen or even lower FiO2 helps maintain tone in the pulmonary microvascularture • Reasonable balance of Qp & Qs include PaO2 about 40mmHg, and systemic diastolic pressure greater than 30mmHg • Ideal time for surgery is about age 3 to 5 days. • It is not uncommon for organ systems to recover fairly rapidly, but then plateau short of complete recovery. Hypoplastic Left Heart Syndrome Preoperative risk factors • The impact of these risk factors on mortality are institution dependent and include noncardiac anomalies or genetic syndromes, lower birth weight, postnatal diagnosis • Additional cardiac risk factors including severe preoperative obstruction to pulmonary venous return, ventricular dysfunction, and moderate to severe atrioventricular valve regurgitation. • Earlier reports found aortic atresia to be a risk factor for death Hypoplastic Left Heart Syndrome Techniques of operation 1. Norwood first-stage procedure Maintain PaCO2 40-45mmHg before operation 2. BCPC as interim procedure 3. Fontan operation 4. Cardiac transplantation Norwood Operation Autologous and interdigitating techniques. Goals of Reconstructive Surgery The overall goal of reconstructive surgery is similar to that for single ventricle physiology, that is , establishment in the neonatal period of an effective mixed circulation in which pulmonary and systemic blood flow are well balanced. • A completely unobstructed systemic arterial pathway from the right ventricle to all organs • A restrictive connection between systemic and pulmonary circulations such that Qp and Qs are adequately balanced • An unobstructed flow of pulmonary venous return across the atrial septum to the right atrium Norwood Operation Surgical Process of HLHS Norwood Operation Results 1. Survival Early death & Time-related survival 2. Incremental risk factors for death 1) No morphologic risk factors 2) Small size of ascending aorta (< 2mm) 3) Tricuspid regurgitation 3. Hemodynamic and morphologic results 1) Nonrestrictive opening between two atria 2) Important tricuspid regurgitation 3) Systolic pressure gradient 4) Most patients have a Qp/Qs of 0.8~1.5, and Pulmonary vascular resistance > 4u ; in 20% Pulmonary artery distortion ; in 12% RV end-diastolic pressure elevation ; in 10% Norwood Operation Pulmonary artery compromise • Mechanisms contributing to pulmonary artery compromise after the Norwood procedure are multiple, and include narrowing at the ductal remnant site, extrinsic compression by the neoaorta or the ductal stump, and distortion by the modified Blalock-Taussig shunt (BTS) insertion. • Particularly at risk for these influences is the retroaortic segment of the common pulmonary artery and the left pulmonary artery (LPA). • Compromised early flow to the LPA may contribute to LPA hypoplasia over time, which can increase morbidity of the subsequent bidirectional cavopulmonary anastomosis & Fontan procedures Modification of Shunt Drawing representing the insertion point of a BTS onto the pulmonary artery premodification (A), and postmodification, with an oblique anastomosis to direct pulmonary blood flow preferentially into the retroaortic pulmonary artery (B). Norwood Operation Survival • Early deaths Myocardial ischemia Imbalance of systemic & pulmonary circulation Subsequent multi-organ failure • Late deaths Not insignificant recently and reflects the delicately balanced nature of circulation Residual defects Norwood Operation Risk factors for deaths 1. Abnormal coronary flow patterns 2. Diminished coronary reserve 3. Neurologic complications 4. Residual lesions 5. Ventricular function Hypoplastic Left Heart Syndrome Results of Fontan operation 1. Survival Early deaths Time-related survival 2. Incremental risk factors for death 1) Tricuspid regurgitation 2) Sudden reduction in ventricular volume immediately after Fontan procedure Indications for Operation • The diagnosis of aortic atresia( hypoplastic left heart physiology) indicates the presence of a fatal disease ; death usually occurs within 1 month of birth and certainly within 1 year. Surgical intervention is therefore advisable unless contraindicated by economic, institutional, legal problems • The treatment begins as soon as the diagnosis has been made, or the child is born. Norwood Procedure MBTS vs RV-PA conduit • Advantages of mBTS include improved pulmonary artery growth owing to the antegrade flow throughout cardiac cycle. • Lower diastolic pressures with the mBTS compromise coronary blood flow & impact cardiac function. • RV-PA conduit provides antegrade flow during systole but diastolic flow reversal, adding to the ventricular volume load & diminished pulmonary artery growth. • MBTS has a recognized incidence of acute shunt occlusion, less common with larger RV-PA conduit. • Ventriculotomy carries an unknown risk for late arrhythmia & diminished ventricular function Norwood Procedure Application of RV-PA conduit • • • • • • Low birth weight Preoperative hypotension Tricuspid regurgitation Pulmonary venous obstruction? Right ventricle dysfunction? Small aorta Norwood Procedure Recent results • It is well demonstrated that single-institution results improve over time independent of significant changes in surgical approach • These improvements are thought to reflect an increased incidence of prenatal diagnosis, surgical experience, improvement in perioperative care with close attention to low cardiac output syndrome, and less tangible human factor. • RV-PA conduit compared with more contemporary mBTS controls showed no difference in survival, but reported a more stable postoperative course RV-PA Conduit RV-PA Conduit RV-PA Conduit Norwood Procedure RV-PA Conduit RV-PA Conduit Procedure 1st_ Stage Reconstruction • Choice of graft ; infant weight more than 3 Kg ; 6mm graft, 2-3Kg ; 5mm graft, less than 2 Kg; 4 mm graft. 50% of the predicted normal size of the main pulmonary artery Hybrid Palliation • Hypoplastic left heart syndrome with aortic atresia is depicted after bilateral pulmonary artery banding (stippled areas) and ductal stenting (crosshatched area). MPA-IA shunt provides source of blood flow to aortic arch (small arrows) in patients in whom retrograde flow across aortic isthmus is obstructed (large arrow). RV-PA Conduit Advantages • This novel procedure may be particularly beneficial to low-birth-weight infants with HLHS. • It would be reproducible for many less experienced surgeons with application of the RV-PA shunt. • The pulmonary bed was not subjected to nor dependent on diastolic flow, and there should be less change in pulmonary blood flow with pulmonary hypertensive crises or during resuscitation • Higher diastolic blood pressures and lower Qp/Qs ratios are associated with a more stable and efficient systemic circulation. • Pulmonary perfusion restricted to systole leads to a lower Qp/Qs ratio and demands more intensive ventilatory support during the first postoperative days. Norwood Procedure Questions in RV-PA conduit • Optimal shunt size and shunt material • Growth & distortion of pulmonary arteries • Effects of the small ventriculotomy on right ventricular function • Potential risk of arrhythmia • Ease of second stage palliation • Potential injury of pulmonic valve • Uncertainty to thrombosis & stenosis Hypoplastic Left Heart Syndrome Special situations & controversies • • • • Aortic atresia with large VSD Borderline hypoplastic left heart syndrome Use or avoidance of circulatory arrest Use of RV-PA conduit Valved conduit Nonvalved conduit Norwood Procedure Laryngopharyngeal dysfunction • Norwood procedure, like other operations on the aortic arch, involves mobilization of the recurrent laryngeal nerve, operative damage to the nerve is one potential source of postoperative swallowing dysfunction. • Postoperative vocal fold paralysis should generally be considered to be permanent dysfunction. • It should be noted that the voice of an infant can improve despite persistence of vocal fold paralysis due to the plasticity of the glottis and the ability of the opposite vocal fold to compensate Swallowing Function Abnormalities • Establishing adequate oral intake is generally the last clinical hurdle to be overcome before hospital discharge • Although we were unable to identify predictors of swallowing dysfunction among our patients, its causes are likely multiple. • Normal infants can have swallowing abnormalities, variability in suck and oral transit time, vallecular residuals after swallowing, & hesitation of bolus transit into the cervical esophagus • Pharyngeal stage of swallowing, controlled by the medulla oblongata, is not well developed in neonates and might contribute to these findings Swallowing Dysfunction Management • Risk factors for dysphagia included age less than 3 years, preoperative intubation, and transesophageal echocardiographic probe in patients less than 5.5 kg. • Routine assessment for swallowing dysfunction and appropriate tailoring of feedings might decrease interstage mortality. • Aspiration is one of the most serious abnormalities of swallowing function • A nasogastric tube, compared with a gastrostomy tube, might be more difficult to manage at home, impair swallowing function and competence of esophageal sphincters, and might worsen gastroesophageal reflux.