* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Powerpoint
Survey
Document related concepts
Artificial gene synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Citric acid cycle wikipedia , lookup
Proteolysis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Blood sugar level wikipedia , lookup
Biosynthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Transcript
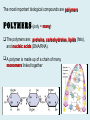
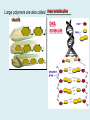
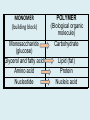
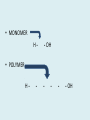
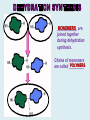
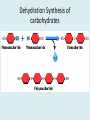
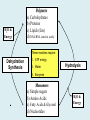
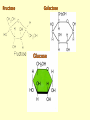
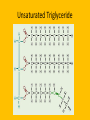
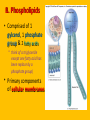
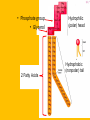
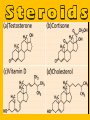
Important Organic Compounds Biological Molecules The most important biological compounds are polymers Polymers (poly = many) many The polymers are: proteins, carbohydrates, lipids (fats), and nucleic acids (DNA/RNA). A polymer is made up of a chain of many monomers linked together moNomers (mono = one) Monomers are: amino acids, sugars, fatty acids, and nucleotides. These are made or broken down over and over in living cells. macromolecules Large polymers are also called _______________ MONOMER (building block) POLYMER (Biological organic molecule) Monosaccharide (glucose) Glycerol and fatty acid Amino acid Nucleotide Carbohydrate Lipid (fat) Protein Nucleic acid • MONOMER H- - OH • POLYMER H- - - - - - OH Process to build polymers • Dehydration synthesis = covalently bonding monomers together by removing water • Called condensation synthesis in text ____________ MONOMERS are joined together during dehydration synthesis. Chains of monomers POLYMERS are called _________ Dehydration Synthesis of carbohydrates To break apart polymers • Hydrolysis: Add H2O H- - - 4H- - - OH + 3H20 - OH • 1 H2O will be consumed for every 1 covalent bond to be broken H 2O & Energy Polymers a) Carbohydrates b) Proteins c) Lipids (fats) d) DNA/RNA (nucleic acids) These reactions require: Dehydration Synthesis 1. ATP energy 2. Water Hydrolysis 3. Enzymes Monomers a) Simple sugars b) Amino Acids c) Fatty Acids & Glycerol d) Nucleotides H2O & Energy Organic molecules: 1. Carbohydrates 2. Lipids 3. Proteins 1. Nucleic Acids **always contains C & H & covalent bonds • C may share electrons with other C 1. Carbohydrates Where does the name come from? Hydrated Carbons: (CH20)n Carbohydrates have the empirical formula of (CH20)n where n = the # of times the chain is repeated. The carbons, hydrogens and oxygens are found in the ratio of 1:2:1 and are made up of a repeating chain of sugars. (CH20)3 = C3H603 (CH20)6 = C6H1206 Sugars are also known as saccarides. saccarides Carbohydrates usually end in ‘ose’. ose Can you think of any examples? The basic sugar molecule is GLUCOSE: GLUCOSE C6 H12 O6. Glucose has a ring structure. Other monosaccharides include fructose, ribose, deoxyribose Three ways to represent the structure of glucose. Actual (molecular) formula for glucose Fructose Galactose Glucose When two sugars bind together via DEHYDRATION SYNTHESIS Examples: – glucose + glucose = maltose + water – glucose + galactose = lactose (milk sugar) + water – glucose + fructose = sucrose (table sugar) + water C12H22011 Complex Carbohydrates = Polysaccharides – Polymer of identical monosaccharides (typically glucose) i. Starch ii. Glycogen i. Cellulose i) Starch • Storage form of glucose in plants (long glucose chains) • Few or no side chains • ii) Glycogen • Animals store their energy (extra glucose) as glycogen in the liver and muscles • Long chains of glucose with many side chains iii) Cellulose • Cell walls of plants are made of cellulose • Long chains of glucose with no side chains and have alternating position of linking oxygen atoms • No mammals can break this bond (indigestible) and serves as fibre Complete carbohydrate review Source Monomer Polymer Animal Plant . Characteristic CH2O (cont`d) • Functions: • Energy for cells • Energy storage (starch, glycogen) • Cell recognition role = glycoprotein or glycolipid • Basis for A, B, and O blood groups 2. Lipids 2. Lipids • Characteristics: – made up of C, H, O (sometimes P or N) – large, insoluble in water • Ex. Water in oil – 2 monomers: 1. Glycerol 2. Fatty acids (long hydrocarbon chains) Lipid Building Blocks Synthesis and degradation of a fat molecule 3 types of Lipids A. Triglycerides • aka neutral fats (nonpolar) or “fats and oils” • 2 Types: a) Fats = animal based & solid (e.g. butter, lard) • 3 Functions: 1. Long term energy source 2. Cushions organs 3. Insulation b)Oil = plant based & liquid (e.g. olive, corn, etc) • Main energy source for plants Triglycerides (cont’d) Structure: – “E” shaped molecule made of one glycerol and 3 fatty acids (synthesized by dehydration of 3 H2O molecules) Triglycerides (cont’d) Structure (cont’d): • Fatty acids are hydrocarbon chains with COOH end – 3 main types of fatty acid 1. Saturated – no C =C which means it is “full” of H, fairly straight, solid at room temp., “fat” (from animals) 2. Unsaturated – at least one C=C bond, structurally bent, liquid at room temp., “oil” (from plants) Fatty Acids Unsaturated Triglyceride Triglycerides (cont’d) Structure (cont’d): • Fatty acids are hydrocarbon chains with COOH end (usually 16-18 carbons) – 3 main types of fatty acid 1. Saturated – no C=C which means it is “full” of H, fairly straight, solid @ room temp., “fat” 2. Unsaturated – at least one C=C bond, structurally bent, liquid at room temp., “oil” 3. Polyunsaturated – many C=C bonds so few H’s (e.g veggie oils) Triglycerides Important - hydrogenation adds H’s (margarine or shortening) but forms trans fats B. Phospholipids • Comprised of 1 gylcerol, gylcerol 1 phosphate group & 2 fatty acids • think of a triglyceride except one fatty acid has been replace by a phosphate group) • Primary components of cellular membranes • Phosphate group • Glycerol 2 Fatty Acids Hydrophilic (polar) head Hydrophobic (nonpolar) tail Phospholipid bilayer Phospholipid bilayer • when placed in H2O, phospholipids form 2 layers – the polar heads (hydrophilic) face out and the non-polar tails (hydrophobic) face in The Cell Membrane C. STEROIDS (lipids) Structure: •4 fused rings of carbon with “stuff” attached (called functional groups) •E.g. Testosterone, estrogen, cholesterol and vitamin D STEROIDS (lipids) Structure (cont`d): •Non-polar so can easily pass through cell membranes •Are water insoluble (it is a lipid) STEROIDS (lipids) Function: •used as sex hormone (e.g. estrogen & testosterone) Emulsification •Needed to mix fats/oils into water •e.g. soap and bile (produced by gallbladder) Review of Carbs & Lipids Quiz 1. Review sheet #s 1-7 2. Inquiry Into Life (12th ed.) online quiz