* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Gene therapy for Leber congenital amaurosis
Public health genomics wikipedia , lookup
Genetic engineering wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Gene expression profiling wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genome (book) wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Gene desert wikipedia , lookup
Gene expression programming wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Gene nomenclature wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Microevolution wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Gene therapy wikipedia , lookup
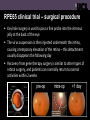
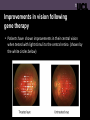
UCL Institute of Ophthalmology Department of Genetics Gene therapy for Leber congenital amaurosis (LCA) caused by damage to the RPE65 gene We conducted the world’s first clinical trial assessing the safety and efficacy of viral gene therapy for inherited sight loss (Bainbridge et al 2008) Leber congenital amaurosis and the RPE65 gene • LCA is a severe inherited retinal disease in which vision is affected from birth and continues to worsen in early adulthood, leading to complete blindness • RPE65 is one of many genes associated with LCA • Damage to the RPE65 gene means that retinal pigment epithelium (RPE) cells underlying the light-sensitive photoreceptor cells cannot recycle the visual pigment used to detect light Why was LCA caused by damage to RPE65 the first eye gene therapy trial? • The genetic cause of the disease is well understood • Although vision is affected early in life and worsens over time, photoreceptor cells remain healthy in the early stages of disease so they can be targeted by gene therapy vectors • We have established proof-of-principle that RPE65 gene replacement restores vision in small and large animal models • The intervention might improve visual function rather than just slow sight loss RPE65 clinical trial – surgical procedure • Key hole surgery is used to pass a fine probe into the vitreous jelly at the back of the eye • The virus suspension is then injected underneath the retina, causing a temporary elevation of the retina – this detachment usually disappears the following day • Recovery from gene therapy surgery is similar to other types of retinal surgery, and patients can normally return to normal activities within 2 weeks Improvements in vision following gene therapy • Several patients have shown improvements in visual function with a variety of tests • Patients have shown improved peripheral vision when tested under dim lighting conditions (shown by the green bars) Improvements in vision following gene therapy • Patients have shown improvements in their central vision when tested with light stimuli to the central retina (shown by the white circles below) Improvements in vision following gene therapy • Some patients have also shown improvements in their ability to navigate through an obstacle course in dark conditions, showing that improvements can be useful in day to day navigation Improved night vision following gene therapy Conclusions and future plans • Retinal gene therapy is safe to perform in the eye • Results so far suggest that it can improve visual function • The success of this trial for LCA paves the way for gene therapy trials for many other types of retinal diseases