* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CLASS NOTES- Balancing Chemical Equations.pptx
Chemical biology wikipedia , lookup
Computational chemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Chemical element wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical bond wikipedia , lookup
Rate equation wikipedia , lookup
American Chemical Society wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Chemical warfare wikipedia , lookup
Process chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Destruction of Syria's chemical weapons wikipedia , lookup
Fine chemical wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Chemical reaction wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Molecular dynamics wikipedia , lookup
Chemical imaging wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Transition state theory wikipedia , lookup
Drug discovery wikipedia , lookup
Safety data sheet wikipedia , lookup
History of molecular theory wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical weapon wikipedia , lookup
History of chemistry wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical industry wikipedia , lookup
Atomic theory wikipedia , lookup
Stoichiometry wikipedia , lookup
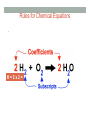
BALANCING CHEMICAL REACTIONS Honors Chemistry Balancing Chemical Equations Learners will know… • The Law of Conservation of Mass as it relates to chemical changes of substances • The parts of a chemical reaction Learners will be able to… • Write and balance chemical equations • Perform stoichiometry calculations The Law of Conservation of Mass • Matter is neither created nor destroyed. • In chemical reactions, the amount of particles in the reactants is equal to the amount of particles in the products. Chemical Reactions • Chemical reactions involve changes in the chemical composition of matter • Creates new materials with new properties • AMOUNT of matter does not change! • Chemical equations describe a chemical reaction • Written similar to a mathematical equation/is like a ‘RECIPE’ Basic Chemical Equations • Reactants = Atoms or compounds that are changed in a chemical reaction (LEFT SIDE) • Products = Atoms or compounds that are generated as a result of a chemical reaction (RIGHT SIDE) Parts of a Chemical Equation Coeffecients and Subscripts • SUBSCRIPTS = how many atoms or ions within a compound • COEFFECIENTS = how many moles or formula units Rules for Chemical Equations • Because of Law of Conservation of Mass, chemical equations MUST BE BALANCED • BALANCED = same number of each kind of atom on both sides (reactants and products) Rules for Chemical Equations • The TOTAL number of any given atom is equal to the COEFFECIENT x SUBSCRIPT H=2x2= 4 Rules for Chemical Equations • You MAY change the COEFFECIENTS • You MAY NOT change the SUBSCRIPTS WHY NOT? Changing the subscripts changes the compound A look at balanced chemical reactions STEPS for Balancing Chemical Equations There are FOUR basic steps 1. Write the correct formula for the reactants and the products ~ DO NOT TRY TO BALANCE IT YET! You must write the correct formulas first. **And most importantly, once you write them correctly DO NOT CHANGE THE FORMULAS! 2. Find the number of atoms for each element on the left side ~ Compare those against the number of the atoms of the same element on the right side. STEPS for Balancing Chemical Equations 3. Determine where to place coefficients in front of formulas ~ Left side must have same number of atoms as the right side for EACH element in order to balance the equation 4. Check your answer to see if: • The numbers of atoms on both sides of the equation are now balanced • The coefficients are in the lowest possible whole number ratios. (reduced) ! Tips and Tricks ! • Take one element at a time, working left to VIDEO: right except for H and O • Try metals then nonmetals • Save H for next to last, and O until last. https:// www.youtube.com/ watch? v=UGf60kq_ZDI • IF everything balances except for O (there is no way to balance O with a whole number) double all the coefficients and try again. (Because O is diatomic as an element) • (Shortcut) Polyatomic ions that appear on both sides of the equation should be balanced as independent units USE THESE STEPS! 1. Metals 2. Nonmetals 3. Hydrogen (H) 4. Oxygen (O) • If Oxygen doesn’t balance, double all coeffecients! EXAMPLE You try it! • Balance the following chemical equation: __C + __S8 à __CS2 TUTORIAL (PHET) • Use the simulation on this site to practice and re-learn the concepts • LINK: http://phet.colorado.edu/blog/2014/09/08/new-html5-balancing-chemicalequations-simulation/ • DIRECT LINK: http://phet.colorado.edu/sims/html/balancing-chemical-equations/latest/ balancing-chemical-equations_en.html MOLE RATIOS